Exhibit 10.2.1
A16138
SECOND AMENDMENT TO THE AMENDED AND RESTATED
STANDARD EXCLUSIVE LICENSE AGREEMENT NO. A5140
WHEREAS, the University of Florida Research Foundation, Inc., a not-for-profit corporation duly organized and existing under the laws of the State of Florida and having its principal office at 223 Grinter Hall, Gainesville, Florida 32611 U.S.A. (hereinafter referred to as “UFRF”), and AxoGen Corporation, a corporation duly organized under the laws of the State of Delaware, and having its principal office at Progress Park, 13631 Progress Blvd. Suite 400, Alachua, FL 32615 (hereinafter referred to as “Licensee”) entered into an Amended And Restated Standard Exclusive License Agreement With Sublicensing Terms effective February 21, 2006 (hereinafter “License Agreement”);
WHEREAS, the parties amended the License Agreement by a Letter Amendment dated on November 10, 2014;
WHEREAS, the parties now wish to further amend the Amended and Restated License Agreement by this Second Amendment (the “Second Amendment”);
WHEREAS, the terms of the November 10, 2014, Letter Amendment do not apply to this Second Amendment;
THEREFORE, in consideration of the premises and mutual covenants contained herein the parties agree as follows.
|
1. |
Licensee shall pay to UFRF an Amendment Fee of Ten Thousand Dollars ($11,000) within thirty (30) days of execution of this Second Amendment. |
|
2. |
The License Agreement shall be amended as follows. |
a.Section 1.1.4 shall be replaced with:
“the United States Patent applications entitled ‘Method for Decellularization of Nerve Allografts,’ filed in the US Patent Office March 15, 2013, serial number 61/794,012 UF docket #14347) and entitled ‘Method for Decellularization of Tissue Grafts’ filed in the US Patent Office September 15, 2015, serial number 14/776,765 UF docket #14347) (the “Section 1.1.4 Patents”).”
b.Section 1.1.5 shall be replaced with:
“all foreign counterparts, and divisionals and continuations both US and foreign, of the patent applications described in sections 1.1.1, 1.1.2, 1.1.3 and 1.1.4 all to the extent owned and controlled by the University of Florida:” and
c.Section 1.1.6 shall be added as follows:
Page 1 of 7
Initials_____
UFRF
A16138
“any reissues and re-examinations of the patents described in sections 1.1.1, 1.1.2, 1.1.3 and 1.1.4.”
d.Section 1.12 shall be replaced with:
“’Licensed Field’ shall be limited to the field of medical devices, human therapeutics and human and animal tissue, and processing of any tissues.”
e.Section 2.2.3 shall be amended to provide an additional last sentence as follows:
If Sublicense of a Licensed Product or Licensed Process from Section 1.1.4 Patents then 20%.
f.The first sentence of Section 3.1.1 will be replaced with the following:
“Licensee agrees to and warrants with respect only to the Section 1.1.4 Patents the following:”
g.Section 3.1.3 including subsections (a) through (i) shall be modified by replacing the first paragraph, including subsections (a) through (i), with the following:
“Licensee agrees that it shall utilize the Licensed Process containing Section 1.1.4 Patents to manufacture a Licensed Product, and/or to provide services to third parties to manufacture products containing Section 1.1.4 Patents for sale pursuant to the timing provided in the Development Plan.”
h.Section 4.3.1 shall be modified by replacing the first paragraph with the following:
“Licensee agrees to pay to UFRF a royalty, on a country-by-country basis, on Net Sales that, but for the license granted under this Agreement, would infringe one or more Valid Claims of Licensed Patents, in such country, as follows:
1)for Licensed Processes and Licensed Products that do not utilize Section 1.1.4 Patents, three percent (3%);
2)for Licensed Processes and Licensed Products, that but for the license granted under this Agreement would infringe one or more Valid Claims, that do utilize Section 1.1.4 Patents, three and three quarter percent (3.75%); and
3)for Licensed Processes and Licensed Products that only utilize Section 1.1.4 Patents, one and three quarter percent (1.75%).
Licensee shall notify UFRF in writing within thirty (30) days when a Licensed Product is manufactured using the Licensed Process containing the Section1.1.4 Patents or when Licensee or its Sublicensee uses the Licensed Process containing the Section 1.1.4 Patents.”
Page 2 of 7
Initials_____
UFRF
A16138
i.Section 4.3.2 shall be amended as follows:
a.New last sentence will be added: “Notwithstanding the foregoing, Licensed Products, Licensed Processes and Licensed Patents shall not include Section 1.1.4 Patents for purposes of this paragraph.”
b.New paragraph will be added as follows:
“Licensee agrees to pay to UFRF a royalty, on a country by country basis, on Net Sales of Licensed Products and/or Licensed Process that utilize Section 1.1.4 Patents that do not infringe any Valid Claims of Licensed Patents in such country, in an amount equal to one percent (1%) of Net Sales of such Licensed Product or Licensed Process in such country by Licensee, its Affiliates, or its Sublicensees, as long as the Licensed Products and /or Licensed Processes are covered by an issued, and unexpired claim, valid in the United States.
c.New paragraph will be added as follows:
“Licensee agrees to pay to UFRF a royalty, on a country by country basis, on Net Sales of Licensed Products and/or Licensed Process that utilize Section 1.1.4 Patents that do not infringe any Valid Claims of Licensed Patents in such country, in an amount equal to one percent (1%) of Net Sales of such Licensed Product or Licensed Process in such country by Licensee, its Affiliates, or its Sublicensees, as long as the Licensed Products and /or Licensed Processes that utilize Section 1.1.4 Patents are covered by an issued, and unexpired claim, valid in the United States and contained in the Section 1.1.4 Patents.
j.Section 4.4.1 shall be amended to replace all but the last paragraphas follows:
In addition to all other payments required under this Agreement, Licensee agrees to pay UFRF Milestone Payments thirty (30) days after the events in the table below as follows:
|
“Payment |
|
Event |
|
$2,000 |
|
FDA approval of Licensee Avance Nerve Graft |
|
|
|
|
|
$25,000 |
|
first commercial use of Licensed Process that utilize Section 1.1.4 Patents to provide services to manufacture products for third parties |
|
|
|
|
|
$10,000 |
|
first use to manufacture Licensed Products that utilize Section 1.1.4 Patents |
Page 3 of 7
Initials_____
UFRF
A16138
Licensee may not deduct the above milestone payments paid to UFRF from any other amounts due under this agreement.”
k.Section 4.4.2 shall be added as follows:
“4.4.2Licensee shall pay an annual license maintenance fee for the Section 1.1.4 Patents of Five Thousand Dollars ($5,000) due on each anniversary date of the execution of this Second Amendment until such time as the Section 1.1.4 Patents are no longer subject to the license provided for in this Second Amendment.”
l.Section 9.3 shall be replaced with the following:
“UFRF may terminate the license to the Patent Rights for the Section 1.1.4 Patents by giving Licensee at least sixty (60) days’ written notice (which shall state UFRF’s intent to terminate such Section 1.1.4 Patents Patent rights and the basis therefor) if commercial development milestones (to the extent relevant) are not satisfied as specified in Section 3.1.3, and if such failure to achieve such milestones was solely due to Licensee’s lack of commercially reasonable diligence in pursuing such milestones. In such event, the license for the Section 1.1.4 Patents rights shall terminate at the end of the notice period specified by UFRF in such notice of termination (at least 60 days) unless (i) the milestone at issue has been achieved prior to the end of such notice period, in which case this Agreement shall continue in full force and effect as to the relevant Section 1.1.4 Patent Rights, or unless (ii) prior to the end of such sixty (60) day period, Licensee disputes in writing that its lack of commercially reasonable diligence was the sole cause of the failure to achieve such milestone and commences the dispute resolution procedures under Section 11. In such case, the license to the Section 1.1.4 Patents (including the parties’ respective rights and obligations hereunder) shall remain in full force and effect until the conclusion of the proceedings described in Sections 11.1 and 11.2 regarding the relevant Section 1.1.4 Patents Patent Rights.”
m.Section 9.4 shall be replaced with the following:
“a. If Licensee at any time defaults in the timely payment of any monies due to UFRF as to the Patent Rights described in Sections 1.1.1, 1.1.2, and 1.1.3 or commits any breach of any other covenant herein contained, except for those related to Section 1.1.4 Patents which are provided for in Section in subsection (b) hereof, and Licensee fails to remedy, or take steps to diligently remedy, any such breach or default within sixty (60) days after written notice thereof by UFRF, UFRF may at its option immediately terminate this Agreement by giving notice of termination to Licensee.
b. If Licensee at any time defaults in the timely payment of any monies due to UFRF or, as to the Section 1.1.4 Patents, in the timely submission to UFRF or any Development Report or fails to actively pursue the Development Plan or commits any breach of any other covenant herein contained, and Licensee fails to remedy, or take steps to diligently remedy, any such breach or default within sixty (60) days after written notice thereof by
Page 4 of 7
Initials_____
UFRF
A16138
UFRF, UFRF may at its option immediately terminate the license to the Section 1.1.4 Patents Patent Rights by giving notice of termination to Licensee.”
n.Section 15.2 shall be replaced with the following:
“If to Licensee:
Corporate Counsel
AxoGen Corporation
13631 Progress Blvd
Suite 400
Alachua, FL 32615
With Copy to:
Attorney
Fahd Riaz
DLA Piper LLP (US)
One Liberty Place
1650 Market Street, Suite 4900
Philadelphia, Pennsylvania 19103-7300
o.Appendix A shall be replaced with the new Development Plan in Appendix A attached to this Second Amendment.
3. All other provisions of the License shall remain in full force and effect and unmodified by this Second Amendment.
4. This Second Amendment shall be effective July 5, 2016
|
UNIVERSITY OF FLORIDA |
|
AxoGen, Inc. |
|
RESEARCH FOUNDATION, INC. |
|
|
|
|
|
|
|
By: /s/David L. Day |
|
By: /s/Karen Zaderej |
|
Name: David L. Day |
|
Name: Karen Zaderej |
|
Title: Director of Technology Licensing |
|
Title: President, CEO, Director |
|
|
|
|
|
Date: July 7, 2016 |
|
Date: July 1, 2016 |
Page 5 of 7
Initials_____
UFRF
A16138
Appendix A – Development Plan
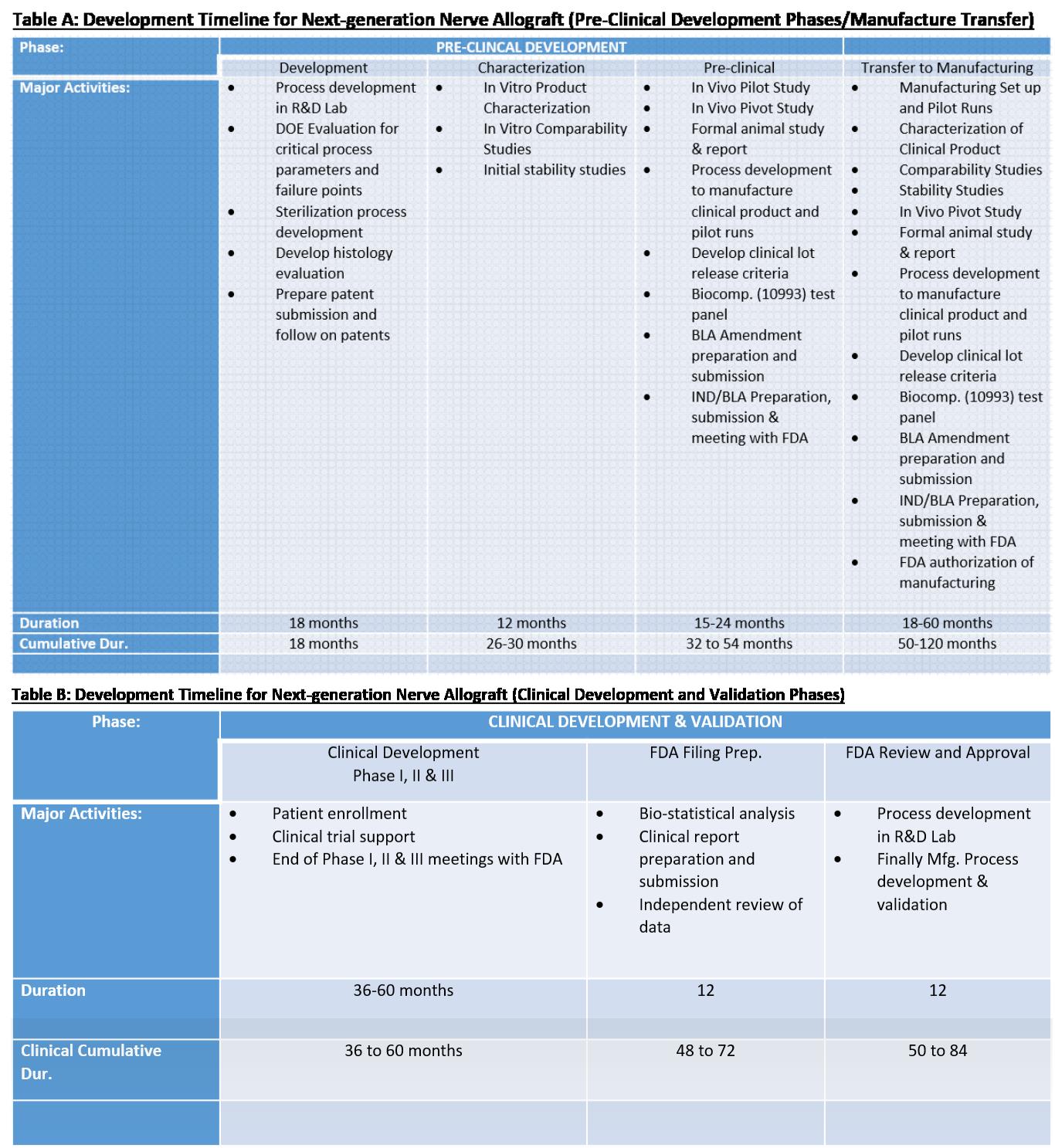
AxoGen is currently the only peripheral nerve tissue processor currently commercializing processed nerve allograft technology. FDA in their December 2014 Minimal Manipulation of Human Cells, Tissues, and Cellular and Tissue-Based Products state that peripheral nerve tissue is a non-structural tissue and that processing that would alter the relevant function of peripheral nerve tissue (in this case, removal of the cellular components) is more than minimally manipulated and should be regulated under a biologic license. AxoGen has an active IND with the FDA Center for Biologics Evaluation and Research for its processed nerve allograft technology and is currently progressing towards a Biological License Application (BLA) submission for Avance® Nerve Graft.
Changes and updates to the manufacturing of Avance® Nerve Graft are subject to review by FDA and depending on the extent of that review may result in minor or major changes to the BLA, or may require an entirely new IND/BLA submission.
To incorporate the UF/Muir processing modifications, AxoGen will follow FDA guidelines for changes to the manufacture of a biological product. Our development plan and timing reflects those and internal requirements to successfully incorporate this into the manufacture of Avance® Nerve Graft.
Table A. reflects the development work and projected timeline for a minor and major change to the FDA regulated process. Table B. reflects the additional timing that maybe required if clinical trials are deemed necessary to establish the performance of the new processing methods. We believe the need for clinical trials is a low risk, but is included as the risk cannot be completely ruled out at this time.
AxoGen will diligently pursue the least timely path to market for this processing change, while maintaining regulatory compliance. This timeline is subject to change.
Page 6 of 7
Initials_____
UFRF
A16138

Page 7 of 7
Initials_____
UFRF