
AXOGEN PROPRIETARY AND CONFIDENTIAL AXOGUARD HA+ NERVE PROTECTOR SUPPLY AND MANUFACTURING AGREEMENT THIS SUPPLY AND MANUFACTURING AGREEMENT (this “Agreement”) is entered into as of the 2nd day of May 2023 (“Effective Date”) by and between Cook Biotech Incorporated, an Indiana corporation having a place of business at 1425 Innovation Place, West Lafayette, Indiana 47906 (“CBI”) and Axogen Corporation, a Delaware corporation, having a place of business at 13631 Progress Blvd., Suite 400, Alachua, FL 32615 (“Axogen”). Axogen and CBI may each be referred to herein as a “Party” and collectively the “Parties.” RECITALS WHEREAS, CBI is engaged in the business of the design, manufacture, sale, and distribution of medical devices and products, including without limitation the Base Component Material (defined below); and WHEREAS, Axogen is engaged in the business of developing, marketing, and distributing medical devices, including without limitation the Device (defined below); and WHEREAS, Axogen and CBI desire to enter into this Agreement whereunder CBI will manufacture, package, and label under the market authorization held by Axogen and exclusively sell to Axogen the Device (defined below) for use in the Field in accordance with and subject to the terms and conditions set forth in this Agreement. NOW, THEREFORE, in consideration of the mutual covenants and agreements herein contained, the Parties mutually agree as follows: Article 1, DEFINITIONS OF CERTAIN TERMS 1.1 “Affiliate” of a Party hereto shall mean any entity that controls or is controlled by such Party or is under common control with such Party. For purposes of this definition, an entity shall be deemed to control another entity if it owns or controls, directly or indirectly, at least fifty percent (50%) of the voting equity of another entity (or other comparable ownership interest for an entity other than a corporation). 1.2 “Field” means Axogen’s exclusive right to import, export, sell, market, advertise, promote and distribute the Device as set forth on Schedule 1 attached hereto. 1.3 “Base Component Material” means a flat sheet of one or more layers of an extracellular matrix (‘‘ECM”) tissue technology covered by United States Patent #8,192,763 and produced by CBI’s proprietary methods and any improvements made thereto, such improvements excluding any coating or other components added to the ECM. 1.4 “Cancellation Fees” shall be the fees payable by Axogen for cancellation of a Firm Purchase Order offset DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 2 of 26 1.5 “Confidential Information” shall mean all information and data provided by one Party to the other Party, including provision to a third party on behalf of the other Party (e.g., FDA or notified bodies) except any portion of such information and data which: (i) is known to the recipient as evidenced by its written records before receipt thereof from the disclosing Party and which is not itself subject to requirements of confidentiality; (ii) is disclosed to the recipient Party or recipients’ designee by a third person who has the right to make such disclosure and which is not itself subject to requirements of confidentiality; (iii) is or becomes part of the public domain through no fault of the recipient; or (iv) the recipient Party can reasonably establish has been independently developed by recipient Party without access to the information disclosed by the disclosing Party. 1.6 “Consumer Price Index” or “CPI” means the consumer price index for all urban consumers as published by the Bureau of Labor Statistics of the U.S. Department of Labor or any successor agency that assumes responsibility for the preparation of such index. 1.7 “Contract Requirements” shall mean 100% of the quantity of a Device or Demo Device used, sold and/or distributed by Axogen in the Territory. 1.8 “Contract Price” shall mean the price paid by Axogen to CBI for each Device or Demo Device that is finished, packaged, labeled, and ready for subsequent sale, distribution, or other use (i.e., Demo Device(s)) by Axogen referenced in Exhibit B and as may be amended by the Parties from time to time. 1.9 “Current Good Manufacturing Practices” or “cGMP” shall mean the good manufacturing practices required by the FDA and set forth in the FD&C Act or FDA Regulations (including without limitation 21 CFR 820), policies or guidelines, in effect at any time during the term of this Agreement, for the manufacture of Devices. 1.10 “Delivery Date” shall mean the date that the Device is delivered to a common carrier chosen by CBI. 1.11 “Demo Device(s)” shall mean a version of the Device prepared for Axogen by CBI intended solely for demonstration that is manufactured or processed in the same manner as a Device; however, it will be: (i) non-sterile; (ii) not cleared for clinical, human use; and (iii) marked “NOT FOR CLINICAL USE.” 1.12 “Device(s)” shall mean the Base Component Material coated on both sides with HA+ Material including but not limited to that which is 4-layer Base Component Material and which is then supplied sterile to Axogen in sterile barrier packaging, product labeling, and outer product carton as set forth on Exhibit “A”. 1.13 “Existing Distribution Agreement” shall mean the Distribution Agreement between Axogen and CBI dated August 27, 2008, as amended, pursuant to which CBI granted to Axogen the exclusive worldwide right to market, sell and distribute certain products utilizing ECM and SIS technology in the “Field” as such term is defined therein currently marketed and sold under Axogen’s Axoguard® family of trademarks DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 3 of 26 1.14 “Effective Date” shall mean the date of this Agreement as set forth above. 1.15 “Existing Products” shall mean those products currently marketed and sold under Axogen’s Axoguard® family of trademarks and subject to the Existing Distribution Agreement. 1.16 “FDA” shall mean the United States Food and Drug Administration or any successor entity thereto. 1.17 “FD&C Act” shall mean the United States Federal Food, Drug and Cosmetic Act, as may be amended from time to time. . 1.18 “HA + Material” means a tissue repair membrane adapted for adhesion and lubrication, including a sodium alginate and sodium hyaluronate coating, encompassed by one or more claims of Axogen’s U.S. Patent Application No. 16/992,857 and any continuations and/or divisionals thereof or related foreign applications, or a patent issuing therefrom, including any improvements thereto made solely and owned solely by Axogen, made solely and owned solely by CBI, or made jointly and owned jointly by Axogen and CBI. 1.19 “Purchase Order” shall mean written orders from Axogen to CBI which shall specify (a) the quantity of Device(s) and Demo Device(s) ordered, (b) the total Contract Price for such order, (c) the requested delivery date(s), and (c) confirmed delivery destination for the order. 1.20 “Regulatory Authority” shall mean any applicable governmental regulatory authority, notified body, or agency involved in granting clearance, authorization, certification, or marketing approvals for the development, manufacture, commercialization, and/or regulation of the Device in the Territory, including without limitation, the FDA in the United States and other national or regional authorities globally. 1.21 “Regulatory Standards” means (a) procurement and maintenance of any and all permits, licenses, filings, certifications required by Regulatory Authority, and compliance with the cGMPs, and (b) any laws, rules, regulations and standards of any Regulatory Authority, (including, without limitation, the Environmental Protection Agency (“EPA”), the Occupational Safety and Health Administration (“OSHA”), the FDA, and state and local authorities), that apply to the Manufacturing Facility or CBI’s manufacturing, storage, handling, or shipment of materials or the Device. 1.22 “Specifications” shall mean, Axogen’s designated written requirements with which a Device must conform as set forth on Exhibit “A” as may be modified subject to the terms of the Quality Agreement. 1.23 “Quality Agreement” shall mean the certain written agreement between CBI and Axogen which outlines each Party’s respective quality and/or regulatory activities and responsibilities related to the Device, as may be amended from time to time. 1.24 “Territory” shall mean worldwide. DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 4 of 26 Article 2, NATURE AND SCOPE OF AGREEMENT 2.1 Nature of Agreement. This Agreement establishes the terms and conditions upon which CBI shall manufacture, package, label, and deliver the Device to Axogen. Pursuant to the terms of this Agreement, Axogen shall have the exclusive right to import, export, sell, resell, market, advertise, promote, and distribute the Device in Axogen’s Field, throughout the Territory. For purposes of clarity, except as expressly set forth in this Agreement with respect to the Device, the terms and conditions of the Existing Distribution Agreement for the Existing Products shall not be modified by this Agreement. 2.2 Axogen Efforts. Axogen shall use commercially reasonable efforts to promote and solicit the sale of the Devices within Axogen’s Field subject to appropriate Regulatory Standards in the Territory and shall not promote or solicit the sale of the Device for use outside of the Field unless agreed to by the Parties in writing via an amendment to this Agreement signed by both Parties. Article 3, PURCHASE AND SUPPLY OF DEVICE 3.1 Agreement to Purchase and Supply. Subject to the terms and conditions of this Agreement, CBI shall manufacture and deliver to Axogen and Axogen shall accept and pay for the Contract Requirements of Devices or Demo Devices pursuant to each accepted applicable Purchase Order. Except as provided herein and during the Term, CBI shall not manufacture or sell to any person other than Axogen or enter into any agreement with any person other than Axogen related to the manufacture or sale of the Device in the Field. For clarity, nothing in this Agreement shall otherwise limit CBI’s right to make, use, sell, offer to sell or import the Base Component Material or products other than the Device, or to use technologies or intellectual property that is or becomes dedicated to the public and which becomes so through no fault or direct or indirect action of CBI. Also, nothing in this Agreement shall otherwise limit Axogen's right to make, use, sell, offer to sell or import the Device or products other than the Device or to use technologies or intellectual property that is or becomes dedicated to the public and which becomes so through no fault or direct or indirect action of Axogen. 3.2 CBI Storage. 3.2.1 Device Storage. In the event CBI stores manufactured Devices in anticipation of future Purchase Orders for such Devices, such storage will be subject to CBI’s standard quality controls and the Quality Agreement including but not limited to the minimum shelf life shipping requirements as set forth in the Specifications; provided, however, in no event shall CBI be required to store the Devices under this Agreement. 3.2.2 Intentionally Omitted. 3.3 Records. CBI shall maintain records and documentation relating to the manufacture and shipment of Devices to Axogen’s designated distribution facility at Burlington, Texas, USA or Alachua, Florida, USA in accordance with cGMP and the Quality Agreement. CBI will make these records available to Axogen subject to the terms of the Quality Agreement. 3.4 CBI shall be deemed to be in default of its supply obligations under this Agreement (a “Supply Default”) if, as clearly and convincingly demonstrated by Axogen: (i) more than two (2) times in any twelve (12) month period CBI fails to fulfill confirmed purchase orders for the Devices from Axogen for a period of more than sixty (60) days or (ii) CBI fails to fulfill confirmed purchase orders for the Devices four (4) times in any continuous twenty four (24) month period, and such failures have caused Axogen to be unable to meet its supply requirements to unaffiliated DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 5 of 26 third parties. In the event of a Supply Default, CBI shall grant to Axogen a contingent right and license to manufacture the Devices for a limited period until as soon as is reasonably practicable after CBI has demonstrated that the circumstance(s) that resulted in the Supply Default have been rectified. Article 4, FORECASTS, ORDERING PROCEDURES, and CAPACITY 4.1 Purchase Orders, Forecasts and Order Limits. Axogen shall provide CBI with a written, rolling forecast of Axogen’s expected monthly purchases of Devices and Demo Devices over the following twelve (12) months. The Parties understand and agree that such forecast is for informational and planning purposes and is solely intended to aid in CBI’s sourcing of materials used in the manufacture of the Device, and both Parties agree that only Purchase Orders accepted as set forth in the Agreement are binding on the Parties. Subsequent to the Effective Date, Axogen will provide CBI with an initial stocking order of Devices and Demo Devices, and thereafter, throughout the Term and subject to the terms of this Agreement, Axogen shall submit to CBI Purchase Orders setting forth the quantities of the Devices and Demo Devices to be purchased by Axogen pursuant to this Agreement, Axogen’s requested delivery date(s), and identifying their Texas or Florida delivery location. 4.2 Acknowledgment of Purchase Orders. Axogen may designate a preferred delivery date for shipment(s) authorized in each Purchase Order, provided final determination and confirmation of delivery dates under each Purchase Order is subject to CBI’s review and acceptance of such Purchase Order, and notification to Axogen thereof. For clarity, requested delivery dates shall not be earlier than thirty (30) calendar days from the date Axogen submits the Purchase Order to CBI, unless approved by CBI in writing. Upon receipt of each Purchase Order, CBI will provide a confirmation of receipt of each Purchase Order to Axogen and confirm the delivery date(s) for such order. Upon Axogen’s receipt of the confirmation, such Purchase Order shall become a “Firm Purchase Order.” In the event CBI is unable to meet a confirmed delivery date and subject to Article 19, CBI shall so notify Axogen and provide to Axogen an alternative delivery date, which shall be as soon as commercially reasonable and practicable and not unreasonably delayed. Notwithstanding anything else contained herein, in no event shall CBI be obligated to accept an order for a quantity of the Device or Demo Device for delivery in any single month that is in excess of two hundred percent (200%) of the average number delivered to Axogen in the three (3) months prior to such single month. Except for modifications or cancellations to a Firm Purchase Order due to non-conformity of the Device or Demo Device, any Firm Purchase Order cancelled in whole or in part without CBI’s written consent is subject to a cancellation fee equal to fifty percent (50%) of the then current Contract Price as set forth in the Purchase Order. To the extent of any conflict between Purchase Orders submitted by Axogen and this Agreement, this Agreement shall control. CBI acknowledges that Axogen shall not be considered to have cancelled a Firm Purchase Order for having given CBI notification of non-conforming Devices pursuant to Article 7.1 below. Article 5, PRICE 5.1 Contract Price. The Contract Price shall be set forth on Exhibit “B,” as may be amended from time to time in accordance with this Agreement and is attached hereto and by reference made a part hereof. 5.2 Contract Price Adjustment. Commencing on the Effective Date of this DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 6 of 26 Agreement the Contract Price for Devices and Demo Devices manufactured by CBI and subsequently shipped to Axogen shall be those as set forth on Exhibit “B”. Pricing shall be adjusted each calendar year of the Agreement as follows: (i) CBI will notify Axogen on or before October 1st of each calendar year of the Contract Price’s new rate for the following year; (ii) such new Contract Price rate increases shall be the lesser of 4% or the percentage increase in the US Consumer Price Index for the most recently published percentage change for the twelve (12) month period preceding September 1st of that calendar year. Commencing on January 1, 2026, the Contract Price rate increases shall be the lesser of 4% or (a) the percentage increase in the US Consumer Price Index for the most recently published percentage change for the twelve (12) month period preceding September 1st of that calendar year and (b) where such percentage increase in the US CPI is greater than 4%, the sum of 4% plus one-half of the difference between such percentage increase in the US CPI and 4% (for example, if such percentage increase in the US CPI is 7%, then the new Contract Price rate increase would be 5.5% -- calculated as 4% + (0.5)(7%- 4%) = 5.5%; or (iii) as may be impacted by a Greater Increase Conditions as set forth below. The new Contract Price for the following calendar year will become effective as of January 1 of the following calendar year. 5.3 During the Term, CBI will notify Axogen of any proposed increases or decreases in the Contract Price for the following calendar year as set forth in Article 5.2 above. In the event there are factors which directly impact CBI’s costs to procure materials, labor, improvement of manufacturing efficiencies, wages, and/or extra or enhanced FDA or other regulatory requirements or amendments in connection with the manufacture and supply of the Devices, such factors will create a Greater Increase Condition (“Greater Increase Condition”). CBI will notify Axogen of such Greater Increase Condition and the nature of its impact; provided, however, CBI is under no obligation to provide CBI’s proprietary information or processes to Axogen for the purposes of Greater Increase Condition review. In no event shall CBI make such an adjustment more than once in any given twelve (12) month period unless CBI demonstrates that a greater increase is described above. Any adjustment arising out of a Greater Increase Condition shall be limited to the direct cost impact experienced by CBI without further mark-up or adjustment. CBI will take commercially reasonable steps to: (i) limit or avoid Greater Increase Conditions; and (ii) consider such Greater Increase Condition when reviewing annual Contract Price review as set forth in Article 5.2 above. 5.4 During the Term, CBI and Axogen shall use commercially reasonable efforts to improve Device manufacturing quality and efficiency, when and where possible. Any cost savings due to improved Device manufacturing efficiency resulting in whole or in part from contributions made by CBI, other than those relating to the manufacture of the Base Component Material itself, shall be equally shared by the Parties. 5.5 Base Component Material Purchase. If at any time during the Term of this Agreement on or after January 1, 2026, Axogen is able to obtain an offer from an unrelated third- party manufacturer to manufacture the Devices or Demo Devices in the United States using Base Component Material purchased from CBI at a price which is lower than the Contract Price then in effect and otherwise under substantially the same terms as this Agreement, Axogen has the right to notify CBI of such offer and request that CBI reduce the Contract Price to meet such lower price. If CBI does not agree to the reduction in the Contract Price within ninety (90) days of receiving Axogen’s request, Axogen shall have the right, but not the obligation, to initiate an Alternate Supply Process by which this Agreement would be terminated and Axogen would enter into an agreement for the manufacturing of the Device and Demo Device with such third-party manufacturer using Base Component Material purchased from CBI. To initiate the Alternate DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 7 of 26 Supply Process, Axogen shall provide CBI written notice thereof, and CBI and Axogen shall enter into good faith negotiations over the ninety (90) day period following such written notice to simultaneously enter into (i) a written supply agreement under which Axogen shall purchase the Base Component Material from CBI for Axogen’s having manufactured the Devices and Demo Devices by such third-party manufacturer, (ii) a written license agreement under which CBI shall grant to Axogen and its third party manufacturer(s) a license on commercially reasonable terms to make, use, sell, offer to sell, import and export the Devices and Demo Devices manufactured by such third party manufacturer incorporating such purchased Base Component Material, and (iii) a written termination of this Agreement. Upon termination of this Agreement as set forth in this Article 5.5, Axogen shall: (i) reimburse CBI for expenses related to cancellation or modification of purchase commitments for materials used by CBI in the manufacture of only the Devices and Demo Devices; (ii) Axogen shall take delivery and shall pay for all open Purchase Orders which have been confirmed by CBI; (iii) Axogen shall reimburse CBI for expenses related to the preparing for shipment and subsequent shipment of materials or Axogen owned equipment to Axogen or their designee. For avoidance of doubt, absent a successful negotiation under this Article 5.5 leading to the execution by the parties of the aforementioned written supply agreement, written license agreement, and written termination agreement, this Agreement shall continue for the Term unless earlier terminated pursuant to Article 8.2. Article 6, SHIPMENT AND INVOICING 6.1 Shipment and Delivery Terms. CBI shall pack and label the Device in accordance with the Specifications and subject to each Purchase Order and place the Devices with a carrier of CBI’s choosing to be delivered on or before the delivery date to either Axogen’s Burlington, Texas, USA or Alachua, Florida, USA facilities CPT (Incoterms, 2020). Title to the Device shipped under any Purchase Order shall pass to Axogen upon delivery of the Device to Axogen pursuant to the terms of this Agreement. 6.2 Payment Terms. CBI may invoice Axogen for each shipment of Devices upon such shipment. Axogen shall pay CBI for each invoice net thirty (30) days from the date of the receipt of such undisputed invoice. Payments not received within the times noted above shall bear interest at the lesser of (a) the maximum rate permitted by law, and (b) 1.5% per month on the outstanding balance compounded monthly. 6.3 Default in Payment Obligations. In addition to all other remedies available to CBI in the event of a Axogen default, if Axogen fails to make payments as required hereunder, CBI may, at its sole election, require one or more of the following: (i) refuse all further Purchase Orders; (ii) refuse to manufacture Devices until Axogen’s account is paid in full; (iii) modify the foregoing terms of payment; (iv) place the account on a letter of credit basis; (v) require full or partial payment in advance; (vi) suspend deliveries of Devices until Axogen provides assurance of performance reasonably satisfactory to CBI; and/or (vii) take other reasonable means as CBI may determine. Article 7, INSPECTION AND ACCEPTANCE OF DEVICE 7.1 Device Conformity. Within thirty (30) calendar days from delivery of a shipment of the Devices or Demo Devices to Axogen, Axogen shall inspect to determine whether Device(s) or Demo Devices in such shipment conform to the Specifications; and shall notify CBI of such inspection and acceptance; provided, however, Axogen shall store all received shipments in DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 8 of 26 compliance to labeled storage requirements prior to such inspection or the improperly stored Devices shall be deemed accepted. Axogen’s failure to notify CBI within the applicable time period of its acceptance of such shipment or rejection of such shipment for a Device or Demo Device that does not conform to the Specifications, then Axogen shall be deemed to have accepted the Device or Demo Device and waived its right to revoke acceptance. 7.1.1 If Axogen stores and handles the Devices in compliance with labeled storage requirements and thereafter reasonably believes any shipment of Device or Demo Device does not conform to the Specifications, including after acceptance as to those Specifications that cannot be inspected for conformity until the Device package is opened (“Post Acceptance Conformity”), it shall notify CBI by telephone including a detailed explanation of the non- conformity and shall confirm such notice in writing via overnight or e-mail delivery to CBI. Upon receipt of such notice, CBI will investigate such alleged non-conformity and reply with suggested correction plan in timeframes set forth in the Quality Agreement, or such timeframe as is reasonably agreed between the Parties. In the event, assertion that CBI disagrees with Axogen’s determination that the shipment of Device is non-conforming, CBI shall promptly notify Axogen by telephone and confirm in writing in material compliance with the terms of the Quality Agreement. 7.1.2 If the Parties dispute whether a shipment of Device is conforming or non- conforming, including Post Acceptance Conformity, the shipment of Device will be submitted to a mutually acceptable laboratory or consultant for resolution, whose determination of conformity or non-conformity, and the cause thereof of non-conformity, shall be binding upon the Parties. The Axogen shall bear the costs of such laboratory or consultant, except as set forth in Article 7.2.3. 7.2 Remedies for Non-Conforming Devices 7.2.1 In the event CBI agrees that the Devices are non-conforming, or the laboratory determines that the Devices are non-conforming, CBI shall replace such non- conforming Devices as soon as commercially practicable. 7.2.2 Axogen shall pay for all Devices, including replacement Devices, except as specifically set forth in Article 7.2.3. In the event CBI agrees, or the laboratory or consultant determines, that the Devices or Demo Devices are nonconforming solely as a result of the negligence or willful misconduct of CBI, then CBI will bear the costs for replacement of each nonconforming Device or Demo Device and bear the costs of such laboratory or consultant. Article 8, TERM AND TERMINATION 8.1 Term. The term of this Agreement commences on the Effective Date and continues in full force and effect until July 1, 2030, unless extended by mutual agreement of the Parties or earlier terminated in accordance with this Article 8 (the “Term”). 8.2 Early Termination. Either Party may terminate this Agreement, upon written notice to the other Party, in the event of any of the following: (i) a breach of any obligation to pay money under this Agreement, unless such obligation is disputed in good faith by the non-paying Party, which breach is not cured within thirty (30) days after receiving written notice of such breach from the non-breaching Party; (ii) a breach of any non-monetary representation or warranty or obligation contemplated in this Agreement, which breach is not cured within sixty (60) calendar days after receiving written notice of such breach from the non-breaching Party; (iii) the other Party’s inability to pay its debts as the same become due; any assignment by the other Party for the benefit of its creditors; the appointment of a receiver, liquidator, or committee of creditors for all or substantially all of the other Party’s business or assets; the filing of a petition for voluntary DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 9 of 26 or involuntary bankruptcy or similar proceeding by or against the other Party or the liquidation of the other Party; (iv) the expropriation, confiscation, or nationalization of all or substantially all of the other Party’s business or assets; or (v) upon the occurrence of any other event constituting grounds for termination set forth in any other provision in this Agreement. 8.3 Additional Rights and Remedies. Termination under this Article 8 shall be in addition to the other rights and remedies of the terminating Party. Termination of this Agreement for any reason shall not relieve any Party of any obligations accruing prior to such termination. 8.4 Non-cancelable Costs and Expenses. In the event of the termination or cancellation of this Agreement, except by Axogen as a result of a breach by CBI under Article 8.2, CBI may submit to Axogen a written notice setting forth the following amounts, in sufficient detail to allow Axogen to review such amounts (a “Termination Claim”): (i) the purchase price under this Agreement for Devices as of the date of termination, not previously paid for, that conform to Specifications and were produced pursuant to this Agreement (including any open and unfulfilled Purchase Orders issued by Axogen and accepted by CBI hereunder); and (ii) CBI’s out-of-pocket costs for unused Device materials ordered by CBI prior to the date of termination and exclusively used in manufacturing the Devices under this Agreement. CBI shall ship Devices and/or those unused materials exclusively used in manufacture of the Devices that Axogen has reimbursed CBI and that are not part of CBI’s proprietary manufacturing methods to Axogen pursuant to Article 6.1. Axogen shall pay CBI amounts due with respect to the Termination Claim, less any amounts owed by CBI to Axogen, if any, within thirty (30) days after Axogen’s receipt of invoice from CBI covering the Termination Claim. Any payment of a Termination Claim will not be deemed a waiver of any of Axogen’s other rights arising under this Agreement or applicable law. A Termination Claim is CBI’s sole remedy for termination of this Agreement under this Article 8.4. Except as expressly provided in this Article 8.4, Axogen will not be liable for and will not be required to make payments to CBI, directly or on account of claims by CBI, for loss of anticipated profit, unabsorbed overhead, facilities and equipment rearrangement costs or rental, unamortized depreciation costs, and general and administrative burden charges. 8.5 Survival. Termination, expiration, cancellation or abandonment of this Agreement through any means or for any reason, except as set forth in Article 8.1, shall be without prejudice to the rights and remedies of either Party with respect to any antecedent breach of any of the provisions of this Agreement. The provisions of Articles 8, 12, 13, 14, 15, 16, 17, and 18 hereof shall survive expiration or termination of this Agreement. 8.6 Effect of Termination or Expiration. The termination of this Agreement will not terminate the Existing Distribution Agreement, or any other agreements or engagements entered into between Axogen and CBI. 8.6.1 Termination by Axogen. Promptly following the effectiveness of a notice of termination delivered by Axogen to CBI hereunder (as stated in such notice), CBI shall, unless otherwise directed by Axogen: (i) promptly terminate all performance under this Agreement and Purchase Orders, such terminations subject to Article 4.2 herein; (ii) transfer title and deliver to Axogen all Devices completed prior to the effectiveness of the notice of termination; and (iii) return to Axogen any property and cease all use of any property including Axogen’s Intellectual Property furnished by or belonging to Axogen, including all Axogen Confidential Information, or dispose of such property in accordance with Axogen’s instructions; provided, however, Axogen shall reimburse CBI for the actual, documented costs associated with such disposal. 8.6.2 Termination by CBI. Promptly following the effectiveness of a notice of termination delivered by CBI to Axogen hereunder (as stated in such notice), Axogen shall, unless DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 10 of 26 otherwise directed by CBI: (i) pay all undisputed outstanding amounts then due and owing to CBI for Devices manufactured pursuant to a Firm Purchase Order prior to the effective date of the notice of termination or within ten (10) business days; (ii) pay all amounts then due and owing to CBI related to any Termination Claim as set forth above; (iii) upon CBI’s return to Axogen of any property furnished by or belonging to Axogen, or CBI’s disposal of the same in accordance with Axogen’s instructions, reimburse CBI for the actual, documented costs associated with such disposal; and (iv) cease all use of CBI’s Intellectual Property, including all CBI Confidential Information and the Applicable CBI Patents. CBI shall transfer title and deliver to Axogen all Devices completed prior to the effectiveness of the notice of termination. 8.6.3 Expiration or termination of the Term will not affect any rights or obligations of the Parties that: (i) come into effect upon or after termination or expiration of this Agreement; or (ii) otherwise survive the expiration or termination of this Agreement as herein set forth. Article 9, MANUFACTURE OF DEVICES 9.1 Manufacture. CBI shall manufacture, package, and label the Devices in accordance with cGMP, the Specifications, the terms of this Agreement, and the Quality Agreement between the Parties, each of which as may be amended from time to time. All quality requirements and regulatory matters shall be set forth in the Quality Agreement and exclusively governed by the Quality Agreement. Manufacturing deviations and investigations which occur during manufacture of Device and which do not cause the manufacture to be non-compliant with cGMP, the Specification, or the 510K clearance for the Device, shall not be deemed to cause such Device to be non-conforming. 9.2 Permits and Licenses. Axogen is the marketing authorization holder of all market authorizations, clearances, registrations, approvals, and listings provided by the Regulatory Authority for the Device in the Territory, except as may be related to CBI’s own approvals, clearances, and facilities authorizations and certifications, and shall have sole responsibility, at its expense, to satisfy and obtain all government and statutory permits, approvals, registrations, certificates and licenses necessary or required for the sale, marketing, and commercialization of the Device manufactured by CBI pursuant to this Agreement. CBI shall have sole responsibility, at its expense, to satisfy, obtain, and maintain all government and statutory permits, approvals, registrations, certificates and licenses required for it to carry out its manufacturing obligations in pursuant to this Agreement. 9.3 Manufacturing Facility. CBI will manufacture the Device solely at one or more of its approved manufacturing facilities subject to the terms of the Quality Agreement. 9.4 Device Specifications. The Specification for the Devices and Demo Devices shall be set forth in writing and agreed between the Parties and referenced by document name and number in Exhibit “A”. Any amendment, addition, or deletion of or to a requirement in the Specifications shall be subject to the requirements of the Quality Agreement. CBI is under no obligation to provide Axogen any other specification for manufacturing equipment or processes, each of which may be proprietary and confidential to CBI. In the event a Regulatory Authority requests or requires access to or review of a specification held by CBI other than a Specification, CBI may respond directly to such Regulatory Authority and provide Axogen with a reference to such information without providing such confidential and proprietary information to Axogen. 9.5 Manufacturing Controls. All manufacturing controls applicable to the manufacture, storage, if any, and shipment of the Devices, including without limitation material DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 11 of 26 storage, design controls, nonconformances and investigations, document retention, and quality audit procedures, shall be subject to the terms and conditions set forth in the Quality Agreement. 9.6 Regulatory Requirements. 9.6.1 Changes to Regulation or Specifications. Each Party promptly shall notify the other of new regulatory requirements of which it becomes aware which are relevant to or materially affect the manufacture, shipment, or subsequent distribution of Devices under this Agreement, and which are required by the FDA, any other applicable Regulatory Authority or other applicable laws or governmental regulations and shall meet and confer with each other with respect to appropriate means to comply with such requirement. 9.6.2 Facility-Specific Changes. If facility, equipment, process or system changes are required of CBI as a result of requirements set forth by the FDA or any other regulatory authority, and such regulatory changes apply primarily to the manufacture of the Device, then Axogen and CBI will review such requirements and agree in writing to an implementation schedule for such regulatory changes and changes to Specifications or Devices. The costs of any such change pursuant to this Article 9.6.2 shall be governed by Article 5.3 of the Agreement. 9.7 Supplied Materials. CBI will be responsible for sourcing the Base Component Material, solutions of sodium hyaluronate and sodium alginate, packaging, and labeling components necessary for manufacture, packaging, and labeling of the Devices to conform to the Specifications. Axogen and CBI shall cooperate with the other and use good faith efforts to secure secondary source materials. Article 10, REGULATORY 10.1 Regulatory Approvals. Axogen will be solely responsible for obtaining all regulatory approvals necessary for the sale, marketing, and distribution of Devices. Subject to CBI’s requirements for maintaining confidentiality of proprietary methods and processes, CBI will endeavor to respond directly to the applicable Regulatory Authority with respect to proprietary information and processes used in the manufacture or processing of the Device or Demo Device, seek confidential treatment of such proprietary information of CBI, and subsequently provide Axogen with a letter of authorization to reference the necessary regulatory documentation required in response to a request for such information by a Regulatory Authority, but without providing such proprietary documentation and information to Axogen. 10.2 Regulatory Authority Communications. Each Party will notify the other pursuant to and subject to the Quality Agreement of any applicable communication, inspection, or interaction directly related to the manufacture, shipment, or distribution of Devices with a Regulatory Authority. Article 11, TRADEMARKS AND PATENTS 11.1 Axogen grants to CBI a non-exclusive, royalty free license to use the Axogen’ s trademarks for the sole purpose of allowing CBI to fulfill its responsibilities under this Agreement. Such license shall not be transferable in whole or in part without prior written approval from Axogen. 11.2 Axogen shall be solely responsible for selecting, registering and enforcing Axogen’s trademarks used to identify the Device and, except as set forth in Article 11.l, shall have sole and exclusive rights in such Axogen Trademarks. 11.3 CBI acknowledges Axogen’s right, title and interest in the trade name that Axogen uses for the Device (provided such trade name does not use or incorporate or adopt any of the DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 12 of 26 Trademarks or CBI’s company names or any confusingly similar word or symbol), its trademarks and trade names listed in product sheets issued by Axogen (collectively ‘‘Axogen Trademarks”), and CBI shall not at any time do or cause to be done any act or thing which directly or indirectly challenges, impairs or adversely affects the Axogen’s Trademarks or Axogen’s goodwill therein. CBI shall not acquire any right, title, or interest in the Axogen Trademarks by virtue of the execution, performance or termination of this Agreement. CBI shall not use any Axogen Trademark, without Axogen’s prior written consent. All goodwill resulting from CBI’s use of the Axogen Trademarks shall inure to the benefit of Axogen. CBI will have no liability under this Agreement for any delay or failure to perform its obligations hereunder to the extent that such delay or failure is due to Axogen’s failure to provide clear, timely and reasonable instructions with respect to CBIs use of Axogen Trademarks in connection with the Device. 11.4 Axogen acknowledges CBI’s exclusive right, title and interest in the trademarks and trade names listed in all catalog and product sheets issued by CBI (collectively “CBI Trademarks”), and Axogen shall not at any time do or cause to be done any act or thing which directly or indirectly challenges, impairs or adversely affects the CBI Trademarks or CBI’s goodwill therein. Axogen shall not acquire any right, title, or interest in the CBI Trademarks by virtue of the execution, performance or termination of this Agreement, except as expressly set forth herein. Axogen shall not use any CBI Trademark, without CBI’s prior written consent. Any goodwill resulting from Axogen’s use of the CBI Trademarks shall inure to the benefit of CBI. Axogen will have no liability under this Agreement for any delay or failure of either Party to perform its obligations hereunder, to the extent that such delay or failure is due to CBI’s failure to timely and reasonably implement Axogen’s instructions with respect to Axogen’s Trademarks or CBI’s Trademarks in connection with the Device. 11.5 Except as provided expressly herein, Axogen may not use the CBI Trademarks or CBI’s name in connection with the importation, marketing, distribution and sale of the Device. Axogen shall not use or adopt the CBI Trademarks or any confusingly similar word or symbol as part of its company name. Axogen shall use CBI’s name or Tradename as follows: limited to “Manufactured by Cook Biotech Inc for Axogen Corporation” CBI Trademarks are to be used. The package label for the Device processed by CBI and delivered pursuant to this Agreement shall be designed by Axogen and follow applicable laws and regulations including stating “Manufactured by Cook Biotech Inc. for Axogen Corporation” or variations thereof in material compliance with applicable Regulatory Standards. Axogen shall be responsible for all costs of repackaging and re- labeling the Device and any revision of the Device brochures and other educational materials, to the extent such repackaging, re-labeling and/or revisions are required due to Axogen Trademark issues that arise with respect to the Device. CBI shall be responsible for all costs of repackaging and relabeling the Device and any revision of the Device brochures and other educational materials, to the extent such repackaging, re-labeling and/or revisions are required due to CBI Trademark or patent marking issues that arise with respect to the Base Component Material or any other CBI material or process. 11.6 Axogen will be solely responsible, at its sole expense, for, and except as otherwise provided in this Agreement, shall have the sole right to make all decisions and determinations with respect to marketing and sales of the Device, all subject to and in compliance with all applicable laws and regulations and the terms and conditions of this Agreement. 11.7 CBI reserves the right to bring such legal action in the courts or administrative agencies of any country within the Territory as may be required to prevent the infringement, imitation, unauthorized sale, purchase or distribution, illegal use, or misuse of the CBI Trademarks or CBI’s name. Axogen promptly shall notify CBI of any infringement, imitation, unauthorized sale, DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 13 of 26 purchase or distribution, illegal use, or misuse of the CBI Trademarks or CBI’s name in connection with the Device and of which Axogen becomes aware, in any manner, and shall render any assistance which CBI may reasonably request in protecting the CBI Trademarks or CBI’s name. 11.8 Axogen reserves the right to bring legal action in the courts or administrative agencies of any country within the Territory as may be required to prevent the infringement, imitation, unauthorized sale, purchase or distribution, illegal use, or misuse of the Axogen Trademarks or Axogen’s name. CBI shall promptly notify Axogen of any infringement, imitation, unauthorized sale, purchase or distribution, illegal use, or misuse of Axogen’s Trademarks or Axogen’s name in connection with the Device or any imitation thereof and of which CBI becomes aware, in any manner, and shall render any assistance which Axogen may reasonably request in protecting the Axogen Trademarks or Axogen’s name. 11.9 Axogen grants to CBI during the Term of this Agreement and in connection with any Device(s) or Demo Device(s) provided to Axogen pursuant to and during the Term of this Agreement, a non-transferable, non-exclusive, royalty free license to and under any and all Applicable Axogen Patents, licenses, trademarks, regulatory data, technical information, know- how, and other Axogen Intellectual Property related to the Device(s), including but not limited to Axogen’s sole or joint rights in any of Axogen’s Existing Intellectual Property as defined in Article 17.1 or in any Foreground Improvements as defined in Article 17.2, solely and to the extent necessary for CBI’s performance under this Agreement and subject to the terms of this Agreement, for the manufacturing of the Device and Demo Device exclusively for Axogen. “Applicable Axogen Patents” shall mean any patent or patent application owned by Axogen including at least one claim that encompasses Devices or their use or manufacture and would be infringed by CBI in performing its obligations or rights under this Agreement absent the license from Axogen under this Article 11.9. Such license(s) shall not be transferable in whole or in part without prior written approval from Axogen. Except for the rights expressly granted, no right, title, or interest of any nature whatsoever is granted under the Applicable Axogen Patents whether by implication, estoppel, reliance, or otherwise, by Axogen to CBI. Accordingly, all rights with respect to any know-how, patent or other intellectual property rights that are not specifically granted herein are reserved by Axogen. 11.10 CBI grants to Axogen and its affiliates, distributors, sub-distributors and successors, during the Term of this Agreement, and in connection with any Devices and Demo Devices provided to Axogen by CBI pursuant to this Agreement or by a third party manufacturer pursuant to Article 3.4 of this Agreement and that may after having been so provided be sold after the Term, a non- transferable, non-exclusive, royalty free license to and under any and all Applicable CBI Patents or other CBI Intellectual Property, including but not limited to CBI’s sole or joint rights in any of CBI’s Existing Intellectual Property as defined in Article 17.1 or in any Foreground Improvements as defined in Article 17.2, to use, offer to sell, sell and import the Devices and Demo Devices supplied to Axogen pursuant to this Agreement in the Field. “Applicable CBI Patents” shall mean any patent or patent application owned by CBI including at least one claim that encompasses Devices or their use or manufacture and would be infringed by Axogen in performing its obligations or rights under this Agreement or by using, offering to sell, selling or importing the Devices and Demo Devices supplied to Axogen pursuant to this Agreement, absent the license from Cook under this Article 11.10. Such license(s) shall not be transferable in whole or in part without prior written approval from CBI. Except for the rights expressly granted, no right, title, or interest of any nature whatsoever is granted under the Applicable CBI Patents whether by implication, estoppel, reliance, or otherwise, by CBI to Axogen. Accordingly, all rights with respect to any know-how, patent or other intellectual property rights that are not specifically granted herein are reserved by CBI. DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 14 of 26 Article 12, REPRESENTATIONS AND WARRANTIES 12.1 Mutual Representations. Each Party hereby represents and warrants to the other Party that (i) the person executing this Agreement is authorized to execute this Agreement; (ii) this Agreement is legal and valid and the obligations binding upon such Party are enforceable by their terms; (iii) each Party has obtained (or will obtain prior to manufacturing or selling or distributing the Devices, as may be applicable to each Party), and will remain in compliance with during the term of this Agreement, all permits, licenses and other authorizations (the “Permits”) which are required under federal, state and local laws, rules and regulations applicable to the manufacture, shipment, sale, or distribution of the Devices, as may be applicable to each Party; and (iv) the execution, delivery and performance of this Agreement does not conflict with any agreement, instrument or understanding, oral or written, to which such Party may be bound, nor violate any law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it. 12.2 CBI Non-Transferable Warranty. CBI warrants that (a) the Devices shall: (i) be manufactured in accordance with cGMP and the Specifications as required by the FDA; (ii) conform to manufacturer’s specifications; (iii) be free from defects in materials and workmanship; and (iv) not be adulterated or misbranded within the meaning of the Federal Food, Drug, and Cosmetic Act as amended; and (v) be delivered free from liens and encumbrances. CBI makes no representation or warranty with respect to printed materials supplied by Axogen or its consignee. No representative of CBI may change any of the foregoing and Axogen accepts the Devices subject to all terms hereof. Axogen acknowledges that the Devices are medical devices that have risks, including those described in its labeling, as used by the FDA. Accordingly, CBI expressly makes no warranties that the Devices will be safe and effective when used, including in each application, in each patient or under any and all circumstances THE FOREGOING WARRANTIES ARE EXCLUSIVE AND IN LIEU OF AND SHALL SUPERSEDE ALL OTHER WARRANTIES OF ANY KIND, WHETHER WRITTEN, ORAL. (b) That CBI has no knowledge of (a) any patents or other intellectual property rights of third parties that would be infringed by CBI’s manufacture of Devices under this Agreement, in particular but not limited to the Base Component Material, or of (b) any proprietary rights of third parties that would be violated by CBI’s performance under this Agreement, and/or by Axogen’s or its affiliates, distributors, sub-distributors or successors’ sale, offer for sale, marketing and/or importation of the Device. (c) that the Component Base Material of the Device shall practice one or more claims of the patents identified in Article 1.3 of this Agreement, including, U.S. Patent No. 8,192,763. CBI further agrees and represents that it shall identify to Axogen any other CBI patents in the U.S. or abroad which the Component Base Material of the Device practices one or more claims of such that those patents may also be identified in virtual or physical patent marking of the Device, and Axogen agrees and represents that it shall virtually or physically patent mark the Devices with at least the U.S. Patents identified by CBI pursuant to this Article 12.2. 12.3 CBI makes no representation or warranty with respect to printed materials supplied by Axogen or its consignee. 12.4 Disclaimer or Warranties. EXCEPT FOR THOSE WARRANTIES SET FORTH IN ARTICLES 12.1, 12.2, and 12.3 OF THIS AGREEMENT, CBI MAKES NO OTHER WARRANTIES, WRITTEN, ORAL, EXPRESS OR IMPLIED, WITH RESPECT TO DEVICES OR THE MANUFACTURE OF DEVICES. ALL OTHER WARRANTIES, EXPRESS OR DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 15 of 26 IMPLIED, INCLUDING, WITHOUT LIMITATION, THE IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE AND HEREBY ARE DISCLAIMED BY CBI. NO WARRANTIES OF CBI MAY BE CHANGED BY ANY REPRESENTATIVES OF CBI. 12.5 Axogen Warranties. Axogen warrants that: (i) it has the right to give CBI any information provided by Axogen hereunder; (ii) that CBI has the right to use such information for the manufacture and shipment of Devices during the Term; (iii) Axogen has no knowledge of any (a) patents or other intellectual rights that would be infringed by CBI’s manufacture of Devices under this Agreement or (b) proprietary rights of third parties which would be violated by CBI’s or Axogen’s performance hereunder and/or by Axogen’s or its affiliates, distributors, sub- distributors or successors’ sale, offer for sale, marketing and/or importation of the Device in the Field. Article 13, LIMITATION OF LIABILITY 13.1 Limitation of Liability. AXOGEN’S SOLE AND EXCLUSIVE REMEDY FOR BREACH OF ARTICLE 7 BY CBI SHALL EITHER REPLACE THE NON-CONFORMING DEVICE OR REIMBURSE AXOGEN FOR THE CONTRACT PRICE FOR THE NON- CONFORMING DEVICE. UNDER NO CIRCUMSTANCES SHALL CBI BE LIABLE FOR LOSS OF USE OR PROFITS OR OTHER COLLATERAL, SPECIAL, CONSEQUENTIAL OR OTHER DAMAGES, LOSSES, OR EXPENSES, INCLUDING BUT NOT LIMITED TO THE COST OF COVER OR THE COST OF A RECALL IN CONNECTION WITH OR BY REASON OF THE MANUFACTURE AND DELIVERY OF DEVICES UNDER THIS AGREEMENT WHETHER SUCH CLAIMS ARE FOUNDED IN TORT OR CONTRACT. THE FOREGOING CONSTITUTES THE SOLE AND EXCLUSIVE REMEDY OF AXOGEN AND THE SOLE AND EXCLUSIVE LIABILITY OF CBI. ALL CLAIMS BY AXOGEN FOR BREACH OR DEFAULT UNDER THIS AGREEMENT SHALL BE BROUGHT WITHIN ONE (1) YEAR AFTER THE CAUSE OF ACTION ACCRUED OR SHALL BE DEEMED WAIVED. CBI’s AGGREGATE LIABILITY TO AXOGEN ARISING OUT OF OR RELATED TO THIS AGREEMENT, WHETHER ARISING OUT OF OR RELATED TO BREACH OF CONTRACT, TORT (INCLUDING NEGLIGENCE), OR OTHERWISE, SHALL NOT EXCEED THE TOTAL AMOUNTS PAID TO CBI BY AXOGEN FOR DEVICES OR DEMO DEVICES PURSUANT TO THIS AGREEMENT (THE “BASE LIABILITY CAP”); PROVIDED, HOWEVER, THAT FOR LIABILITY FOR BREACH OF CONFIDENTIALITY OR LIABILITY FOR INFRINGEMENT OR MISAPPROPRIATION OF INTELLECTUAL PROPERTY RIGHTS, CBI’s AGGREGATE LIABILITY SHALL NOT EXCEED TWO (2) TIMES THE BASE LIABILITY CAP; AND, FURTHER PROVIDED THAT LIABILITY FOR INDEMNIFICATION SHALL BE EXCEPTED FROM THIS PARAGRAPH. Article 14, INDEMNIFICATION 14.1 Axogen Indemnification. Axogen shall indemnify, defend and hold harmless CBI and its directors, officers, employees, subcontractors, agents and Affiliates (“Indemnified CBI Parties”) from and against any and all liabilities, obligations, penalties, claims, judgments, demands, actions, disbursements of any kind and nature, suits, losses, damages, costs and expenses (including, without limitation, reasonable attorney’s fees) arising out of or in connection with DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 16 of 26 property damage or personal injury (including without limitation death) of third parties (collectively “Claims”) referring to, relating to or based on: (i) Axogen’s storage, promotion, labeling, marketing, distribution, use or sale of Devices or products incorporating Devices; (ii) Axogen’s violation of any regulatory rules, regulations or laws relating to the sale, marketing or distribution of Devices or products incorporating Devices, including regulations governing anti- kickback and corruption; (iii) Axogen’s negligence or willful misconduct, (iv) a material breach of this Agreement by Axogen, (v) any claim that the use, sale, manufacture, marketing or distribution of Devices or products incorporating Devices by CBI or Axogen, based solely on the HA+ Material itself or as a device coating, violates the intellectual property rights of any third party, or (vi) any claim of trademark infringement due to the sale of the Devices and solely related to Axogen’s Trademarks, except to the extent any of the foregoing is caused solely by the negligence or willful misconduct of the Indemnified CBI Parties 14.2 CBI Indemnification. CBI shall indemnify, defend and hold harmless Axogen and its directors, officers, employees, subcontractors, agents and Affiliates (“Indemnified Axogen Parties”) from and against any and all Claims relating, referring, or based on : (i) any non- conformance of the Device with its then-current Specifications and/or Quality Agreement when delivered to Axogen, including but not limited to any requirements of the Specifications and/or Quality Agreement relating to CBI’s storage of any Device or precursor thereto, including the Base Component Material, or any component thereof, or to any regulatory labeling requirement set forth by a Regulatory Authority for which CBI is responsible; (ii) a failure by CBI to manufacture or deliver a Device in material compliance with applicable regulatory law, rule, or regulation; or (iii) the negligent act or omission or willful misconduct of CBI; (iv) a material breach of this Agreement by CBI, or (v) the assertion of third-party intellectual property rights based solely on the Base Component Material; the assertion of false patent marking based on virtual patent marking of the Device under any CBI patent, including as identified in Article 1.3 of this Agreement, and discussed in Article 12.3 of this Agreement, except to the extent any of the foregoing is caused solely by the negligence or willful misconduct of the Indemnified Parties or solely by the breach by Axogen of its obligations under this Agreement or (vi) any claim of trademark infringement due to the sale of the Devices and solely related to CBI’s Trademarks, except to the extent any of the foregoing is caused solely by the negligence or willful misconduct of the Indemnified Axogen Parties or solely by the breach by Axogen of its obligations under this Agreement. 14.3 Indemnitee Obligation. A Party (the “Indemnitee’’) which intends to claim indemnification under this Article 14 shall promptly notify the other Party (the “Indemnitor”) in writing of any action, claim or other matter in respect of which the Indemnitee or other of its Affiliates, or any of their respective directors, officers, employees, subcontractors, or agents, intend to claim such indemnification; provided, however, that failure to provide such notice within a reasonable period of time shall not relieve the Indemnitor of any of its obligations hereunder except to the extent the Indemnitor is prejudiced by such failure. The Indemnitee shall permit, and shall cause its Affiliates, and their respective directors, officers, employees, subcontractors and agents to permit, the Indemnitor, at its discretion, to settle any such action, claim or other matter, and the Indemnitee agrees to the complete control of such defense or settlement by the Indemnitor. Notwithstanding the foregoing, the Indemnitor shall not enter into any settlement that would adversely affect the Indemnitee’s rights hereunder, or impose any obligations on the Indemnitee in addition to those set forth herein, in order for it to exercise such rights, without Indemnitee’s prior written consent, which shall not be unreasonably withheld or delayed. No such action, claim or other matter shall be settled without the prior written consent of the Indemnitor, which shall not DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 17 of 26 be unreasonably withheld or delayed. The Indemnitee, its Affiliates, and their respective directors, officers, employees, subcontractors and agents shall fully cooperate with the Indemnitor and its legal representatives in the investigation and defense of any action, claim or other matter covered by the indemnification obligations of this Article 14. The Indemnitee shall have the right, but not the obligation, to be represented in such defense by counsel of its own selection and at its own expense. Article 15, INSURANCE 15.1 Axogen Insurance. Axogen shall procure and maintain during the Term of this Agreement and for a period forty-eight (48) months beyond the expiration date of the last Device delivered by CBI to Axogen pursuant to the final Purchase Order fulfilled under this Agreement, Commercial General Liability Insurance, including without limitation, Product Liability and Contractual Liability coverage (the “Axogen Insurance”). The Axogen Insurance shall cover amounts not less than ten million dollars ($10,000,000) combined single limit and shall be with an insurance carrier reasonably acceptable to CBI. CBI shall be named as an additional insured on the Axogen Insurance and Axogen promptly shall deliver a certificate of Axogen Insurance and endorsement of additional insured to CBI evidencing such coverage. If Axogen fails to furnish such certificates or endorsements, or if at any time during the Term of this Agreement CBI is notified of the cancellation or lapse of the Axogen Insurance, and Axogen fails to rectify the same within ten (10) calendar days after notice from CBI, in addition to all other remedies available to CBI hereunder, CBI, at its option, may obtain the Axogen Insurance and Axogen promptly shall reimburse CBI for the cost of the same. Any deductible and/or self insurance retention shall be the sole responsibility of Axogen. 15.2 CBI Insurance. CBI shall procure and maintain during the Term of this Agreement and for a period forty-eight (48) months beyond the expiration date of the last Device delivered pursuant to the final Purchase Order under this Agreement, Commercial General Liability Insurance, including without limitation, Product Liability and Contractual Liability coverage (the “CBI Insurance”). The CBI Insurance shall cover amounts not less than ten million dollars ($10,000,000) combined single limit. CBI promptly shall deliver a certificate of CBI Insurance to Axogen evidencing such coverage. Any deductible and/or self insurance retention shall be the sole responsibility of CBI. Article 16, RECALL OF DEVICE 16.1 In the event Axogen shall be required to recall any Device because such Device may violate local, state or federal laws or regulations, the laws or regulations of any applicable foreign government or agency or the Specifications, or in the event that Axogen or CBI elects to institute a voluntary recall, Axogen shall be responsible for coordinating such recall. Axogen promptly shall notify CBI if any Device is the subject of a recall and provide CBI with a copy of all documents relating to such recall. CBI shall cooperate with Axogen in connection with any recall, at Axogen’s expense except as otherwise provided herein. Article 17, INTELLECTUAL PROPERTY 17.1 Existing Intellectual Property. Except as the Parties may otherwise expressly agree in writing, each Party shall continue to own its patents, trademarks, copyrights, trade secrets, DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 18 of 26 know-how, and other intellectual property existing as of the Effective Date of this Agreement, without conferring any interests therein on the other Party. Without limiting the generality of the preceding sentence, CBI shall retain all right, title and interest to its patents, trademarks, copyrights, trade secrets, know-how, and other intellectual property existing as of the Effective Date of this Agreement, including that which is related to the HA+ Material, arising under the United States Patent Act, the United States Trademark Act, the United States Copyright Act and all other applicable laws, rules and regulations (collectively, “CBI’s Existing Intellectual Property”). Without limiting the generality of the first sentence, Axogen shall retain all right, title and interest to its patents, trademarks, copyrights, trade secrets, know-how, and other intellectual property existing as of the Effective Date of this Agreement, including that which is related to the HA+ Material, arising under the United States Patent Act, the United States Trademark Act, the United States Copyright Act and all other applicable laws, rules and regulations, (collectively, “Axogen’s Existing Intellectual Property”). Neither Axogen or any third party shall acquire any right, title or interest in CBI’s Existing Intellectual Property by virtue of this Agreement or otherwise, except to the extent expressly provided herein. Neither CBI or any third party shall acquire any right, title or interest in Axogen’s Existing Intellectual Property by virtue of this Agreement or otherwise, except to the extent expressly provided herein. For avoidance of doubt, CBI agrees that nothing in this Agreement transfers, or obligates the transfer of, any of Axogen’s Existing Intellectual Property to CBI, including but not limited to Axogen’s Existing Intellectual Property governed by the provisions of the Master Development Agreement between the parties dated November 26, 2019 (the “MDA”); and, Axogen agrees that nothing in this Agreement transfers, or obligates the transfer of, any of CBI’s Existing Intellectual Property to Axogen, including but not limited to CBI’s Existing Intellectual Property governed by the provisions of the MDA. Notwithstanding anything to the contrary in this or any other agreement between the Parties, as between Axogen and CBI, the Parties agree that Axogen solely owns all right, title, and interest in and to US Patent Application No. 16/1992,857 and any continuations and/or divisional thereof or priority-related foreign applications and any patents issuing therefrom. 17.2 Foreground Improvements. CBI and Axogen acknowledge and agree that any rights to intellectual property made, developed, or conceived by Axogen on or after the Effective Date in performing under this Agreement, including that which is related the HA+ Material, shall be owned by Axogen and shall be subject to Axogen’s grant pursuant to Article 11.9. CBI and Axogen acknowledge and agree that any rights to intellectual property made, developed, or conceived by CBI on or after the Effective Date in performing under this Agreement, including that which is related to the HA+ Material, shall be owned by CBI and shall be subject to CBI’s grant pursuant to Article 11.10. 17.3 Disclaimer. Except as otherwise expressly provided herein, nothing contained in this Agreement shall be construed or interpreted, either expressly or by implication, estoppel or otherwise, as: (i) a grant, transfer or other conveyance by either Party to the other of any right, title, license or other interest of any kind in any of its Intellectual Property, (ii) creating an obligation on the part of either Party to make any such grant, transfer or other conveyance or (iii) requiring either Party to participate with the other Party in any cooperative development program or project of any kind or to continue with any such program or project. 17.4 Rights in Intellectual Property. The Party owning any Intellectual Property shall have the worldwide right to control the drafting, filing, prosecution and maintenance of patents relating to such Intellectual Property, including decisions about the countries in which to file patent applications. Patent costs associated with the patent activities described in this Article shall be borne by the sole owner. Each Party will cooperate with the other Party in the filing and DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 19 of 26 prosecution of patent applications. Such cooperation will include, but not be limited to, furnishing supporting data and affidavits for the prosecution of patent applications and completing and signing forms needed for the prosecution, assignment and maintenance of patent applications. 17.5 Confidentiality of Intellectual Property. Intellectual Property shall be deemed to be the Confidential Information of the Party owning such Intellectual Property unless excepted from Confidential Information pursuant to Article 1.5. The protection of each Party’s Confidential Information is described in Article 17. Any disclosure of information by one Party to the other under the provisions of this Article 17 shall be treated as the disclosing Party’s Confidential Information under this Agreement unless excepted from Confidential Information pursuant to Article 1.5. It shall be the responsibility of the Party preparing a patent application to obtain the written permission of the other Party to use or disclose the other Party’s Confidential Information in any patent application before the application is filed and for other disclosures prior to them being made during the prosecution of the patent application. Article 18, CONFIDENTIAL INFORMATION, NONDISCLOSURE, AND PUBLICITY 18.1 Confidentiality. It is contemplated that in the course of the performance of this Agreement each Party may, from time to time, disclose Confidential Information to the other, but is under no obligation to disclose Confidential Information. Each Party agrees to take all reasonable steps to prevent improper or unauthorized disclosure of Confidential Information to third parties. No provision of this Agreement shall be construed so as to preclude disclosure of Confidential Information as may be reasonably necessary to secure from any governmental agency necessary approvals or licenses or to obtain patents with respect to the Device; provided, however, the disclosing Party may limit such disclosure to the other Party to reasonably protect sensitive or proprietary information, and the disclosing Party may seek from the applicable governmental agency or Regulatory Authority appropriate protection of its Confidential Information. 18.2 Litigation and Governmental Disclosure. Each Party may disclose Confidential Information hereunder to the extent such disclosure is reasonably necessary for prosecuting or defending litigation, complying with applicable governmental regulations, apply for Regulatory Approval in the Territory, or conducting pre- clinical or clinical trials, provided that if a Party is required by law or regulation to make any such disclosure of the other Party’s Confidential Information it will, except where impractical for necessary disclosures, for example in the event of a medical emergency, give reasonable advance notice to the other Party of such disclosure requirement and will use good faith efforts to assist such other Party to secure a protective order or confidential treatment of such Confidential Information required to be disclosed. 18.3 Limitation of Disclosure. The Parties agree that, except as otherwise may be required by applicable laws, regulations, rules or orders, including without limitation the rules and regulations promulgated by the United States Securities and Exchange Commission, and except as may be authorized in Article 18.4, no information concerning this Agreement and the transactions contemplated herein shall be made public by either Party without the prior written consent of the other. 18.4 Publicity and SEC Filings. The Parties agree that the public announcement of the execution of this Agreement shall only be by one or more press releases mutually agreed to by the Parties. The failure of a Party to return a draft of a press release with its proposed amendments or modifications to such press release to the other Party within five (5) days of such Party’s receipt of such press release shall be deemed as such Party’s approval of such press release as received by such Party. Each Party agrees that it shall cooperate fully and in a timely manner with the other with DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 20 of 26 respect to all disclosures to the Securities and Exchange Commission and any other governmental or regulatory agencies, including requests for confidential treatment of Confidential Information of either Party included in any such disclosure. 18.5 Duration of Confidentiality. All obligations of confidentiality and non-use imposed upon the Parties under this Agreement shall expire ten (10) years after the expiration or earlier termination of this Agreement; provided, however, that Confidential Information which constitutes the trade secrets of a Party shall be kept confidential indefinitely, subject to the limitations set forth in Articles 18.3 through 18.5. Article 19, FORCE MAJEURE 19.1 Any delay in the performance of any of the duties or obligations of either Party hereto (except the payment of money) caused by an event outside the affected Party’s reasonable control shall not be considered a breach of this Agreement, and unless provided to the contrary herein, the time required for performance shall be extended for a period equal to the period of such delay. Such events shall include without limitation, acts of God; acts of public enemies; epidemic/pandemic; insurrections; riots; injunctions; embargoes; labor disputes, including strikes, lockouts, job actions, or boycotts; fires; explosions; floods; shortages of material or energy; delays in the delivery of raw materials; acts or orders of any government or agency thereof or other unforeseeable causes beyond the reasonable control and without the fault or negligence of the Party so affected. The Party so affected shall give prompt notice to the other Party of such cause and a good faith estimate of the continuing effect of the force majeure condition and duration of the affected Party’s nonperformance and shall take whatever reasonable steps are appropriate to relieve the effect of such causes as rapidly as possible. If the period of nonperformance by CBI because of CBI force majeure conditions exceeds ninety (90) calendar days, Axogen may terminate this Agreement by written notice to CBI. If the period of nonperformance by Axogen because of Axogen force majeure conditions exceeds ninety (90) calendar days, CBI may terminate this Agreement by written notice to Axogen. Article 20, NOTICES 20.1 All notices and other communications in connection with this Agreement shall be in writing and shall be sent to the respective Parties at the following addresses, or to such other addresses as may be designated by the Parties in writing from time to time in accordance with this Article, by registered or certified mail, postage prepaid, or by overnight courier service, service fee prepaid, or by electronic mail with a hard copy to follow via overnight courier service in accordance with this Article. DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 21 of 26 If to CBI: Cook Biotech Incorporated 1425 Innovation Place West Lafayette, IN 47906 Attn: President With a copy to: Cook Group Incorporated 750 Daniels Way Bloomington, IN 47404 Attn: General Counsel If to Axogen: Axogen Corporation 13631 Progress Blvd., Suite 400 Alachua, FL 32615 Attn: General Counsel Notices shall be deemed effective upon receipt. A Party may change its address listed above by notice to the other Party given in accordance with this Article. Article 21, APPLICABLE LAW 21.1 This Agreement is being delivered and executed in the State of Indiana. In any action brought regarding the validity, construction and enforcement of this Agreement, it shall be governed in all respects by the laws of the State of Indiana, without regard to the principals of conflicts of laws. The courts of the State of Indiana shall have personal jurisdiction over the Parties hereto in all matters arising hereunder, and venue for such suit will be in a state of federal court for the City of Bloomington, Indiana. Article 22, ASSIGNMENT 22.1 Neither CBI nor Axogen may assign or transfer this Agreement or any of its rights or obligations hereunder without the prior written consent of the other Party. Notwithstanding the foregoing, either Party, without the consent of the other Party, may assign this Agreement and all of its rights or obligations hereunder to a successor or an Affiliate, or in connection with a merger or sale of all or substantially all of the stock or assets of such assigning Party to which this Agreement pertains; provided, that such assignee shall be obligated in writing to assume all of the assignor’s obligations hereunder. Article 23, TAXES 23.1 Axogen shall pay all national, state, municipal or other sales, use, excise, import, value added, or other similar taxes, assessments or tariffs assessed upon or levied against the sale of Device to Axogen pursuant to this Agreement or the sale or distribution of Device by Axogen (or at Axogen’s sole expense, defend against the imposition of such taxes and expenses). CBI shall notify Axogen of any such taxes that any governmental authority is seeking to collect from CBI, and Axogen may assume the defense thereof in CBI’s name, if necessary, and CBI agrees to fully DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 22 of 26 cooperate in such defense to the extent of the capacity of CBI, at Axogen’s expense. CBI shall pay all national, state, municipal or other taxes to the extent due or attributable on the income resulting from the sale by CBI of the Device to Axogen under this Agreement, including but not limited to, gross income, adjusted gross income, supplemental net income, gross receipts, excess profit taxes, or other similar taxes. Article 24, SUCCESSORS AND ASSIGNS 24.1 This Agreement shall be binding upon and shall inure to the benefit of the Parties hereto, their successors and permitted assigns. Article 25, ENTIRE AGREEMENT 25.1 This Agreement constitutes the entire agreement between the Parties concerning the subject matter hereof and supersedes all written or oral prior agreements or understandings with respect thereto. This Agreement may be executed in counterparts, each of which shall be deemed to be an original and all of which together shall be deemed to be one and the same instrument. Article 26, SEVERABILITY 26.1 If any term or provision of this Agreement shall for any reason be held invalid, illegal or unenforceable in any respect, such invalidity, illegality or unenforceability shall not affect any other term or provision hereof, and this Agreement shall be interpreted and construed as if such term or provision, to the extent the same shall have been held to be invalid, illegal or unenforceable, had never been contained herein. Article 27, WAIVER AND MODIFICATION OF AGREEMENT 27.1 No waiver or modification of any of the terms of this Agreement shall be valid unless in writing and signed by authorized representatives of both Parties hereto. Failure by either Party to enforce any rights under this Agreement shall not be construed as a waiver of such rights nor shall a waiver by either Party in one or more instances be construed as constituting a continuing waiver or as a waiver in other instances. Article 28, INDEPENDENT CONTRACTOR 28.1 Nothing contained in this Agreement shall be construed to constitute either Party as a partner, employee or agent of the other Party, nor shall either Party hold itself out as such. Except as otherwise provided herein, each Party shall be responsible for its own expenses incidental to the performance of its obligations hereunder. CBI shall act as an independent contractor for Axogen in providing the services required hereunder and shall not be considered an agent or joint venturer with Axogen. [SIGNATURE PAGE BELOW] DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 23 of 26 IN WITNESS WHEREOF, the Parties have caused this Agreement to be signed by their duly authorized representatives as of the date written below. “CBI” “Axogen” COOK BIOTECH INCORPORATED AXOGEN CORPORATION By: By: Name: Name: Title: Title: Date: Date: DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049 5/2/2023 Chairman, CEO and President Karen Zaderej 5/3/2023 Umesh Patel President

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 24 of 26 SCHEDULE 1 Axogen’s Field 1. Clinical Applications – uses in the Peripheral Nervous System and the Central Nervous System throughout the human body, including any combinations thereof, but expressly excluding dura mater repair and the oral cavity for endodontic and periodontal applications and oral and maxillofacial surgery solely as it relates to dental, soft or hard, tissue repair and/or reconstruction. 2. Disease States – (a) Nerve Compressions; (b) Nerve, Spinal Transections, Repair and Regeneration; (c) Neuromas; (d) Acute and Chronic Neuropathic Pain (i.e. pain caused by damage or disease of the somatosensory nervous system), including any combinations thereof, all within the Clinical Applications. DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049

AXOGEN PROPRIETARY AND CONFIDENTIAL Page 25 of 26 Exhibit “A” Device and Specifications “Device” shall mean the Base Component Material coated on both sides with HA+ Material including but not limited to that which is 4-layer Base Component Material and which is then supplied sterile to Axogen in sterile barrier packaging, product labeling, and outer product carton Specifications: The Based Component Material shall be a 4-layer Base Component Material; the Base Component material shall have been vacuum-pressed; the Base Component Material shall have been, after it is vacuum-pressed, coated on both sides with the HA+ Material; the Device shall be for use in Axogen’s Field; the outer product carton shall bear the identifier “Axoguard HA+ Nerve Protector”; further specification will be as provided in the authorized specifications as set forth in the Pre-market clearance and maintained in the Quality Agreement, and as may be amended or changed subject to the controls of the Quality Agreement from time to time. DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049
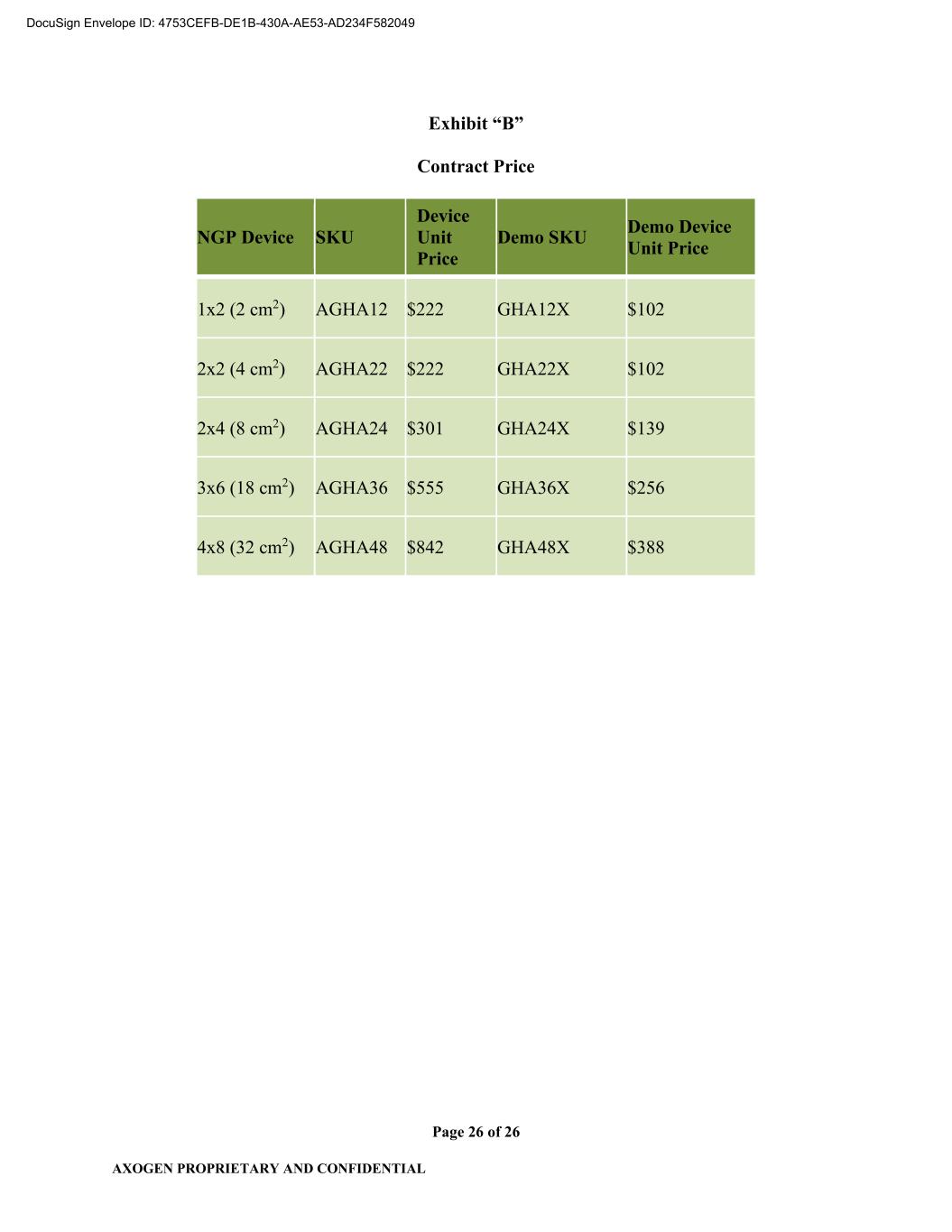
AXOGEN PROPRIETARY AND CONFIDENTIAL Page 26 of 26 Exhibit “B” Contract Price NGP Device SKU Device Unit Price Demo SKU Demo Device Unit Price 1x2 (2 cm2) AGHA12 $222 GHA12X $102 2x2 (4 cm2) AGHA22 $222 GHA22X $102 2x4 (8 cm2) AGHA24 $301 GHA24X $139 3x6 (18 cm2) AGHA36 $555 GHA36X $256 4x8 (32 cm2) AGHA48 $842 GHA48X $388 DocuSign Envelope ID: 4753CEFB-DE1B-430A-AE53-AD234F582049