00008059282022FYfalse00008059282022-01-012022-12-3100008059282022-06-30iso4217:USD00008059282023-03-10xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________________________________________________________________
Form 10-K/A
Amendment No. 1
___________________________________________________________________________
(Mark One)
| | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2022
Or
| | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ____________ to______________
Commission File Number: 001-36046
AXOGEN, INC.
(Exact name of registrant as specified in its charter)
Minnesota
(State or other jurisdiction of
incorporation or organization)
13631 Progress Blvd., Suite 400 Alachua, FL
(Address of principal executive offices)
41-1301878
(I.R.S. Employer
Identification No.)
32615
(Zip Code)
Registrant’s telephone number, including area code: (386) 462-6800
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of exchange on which registered |
| Common Stock, $0.01 par value | | AXGN | | The Nasdaq Stock Market |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐
Non-accelerated filer ☐
Accelerated filer x
Smaller reporting company ☐
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. □
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). □
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
As of June 30, 2022, the last day of the registrant's most recently completed second quarter, the aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was approximately $221,521,729 based upon the last reported sale price of the common stock on the Nasdaq Capital Market.
The number of shares outstanding of the Registrant’s common stock as of March 10, 2023, was 42,601,918 shares.
PCAOB ID: 34 Auditor Name: Deloitte & Touche LLP Auditor Location: Miami FL
DOCUMENTS INCORPORATED BY REFERENCE
None.
EXPLANATORY NOTE
Axogen, Inc. is filing this Amendment No. 1 (the “Amendment No. 1”) to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022 (the “Original Form 10-K”), filed with the Securities and Exchange Commission (the “SEC”) on March 14, 2023, the "Original Filing Date".
This Amendment No. 1 (i) amends and restates Part II, Item 5 to add the Stock Performance Graph, Part III, Items 10, 11, 12, 13, and 14, and amends Part IV, Item 15 of the Original Form 10-K and (ii) deletes the reference on the cover of the Original Form 10-K to the incorporation by reference of portions of our proxy statement into Part III of the Original Form 10-K and (iii) includes the signatures from our directors and Chief Executive Officer , which were inadvertently omitted in the Original 10-K.
In addition, pursuant to SEC rules, Item 15 of Part IV of the Original Form 10-K is hereby amended solely to include, as Exhibits 31.3 and 31.4, new certifications of our principal executive officer and principal financial officer pursuant to Rule 13a-14(a) under the Exchange Act. Because no financial statements are included in this Amendment No. 1 and this Amendment No. 1 does not contain or amend any disclosure with respect to Items 307 and 308 of Regulation S-K, paragraphs 3, 4, and 5 of such certifications have been omitted. We are not including new certifications required by Rule 13a-14(b) under the Exchange Act as no financial statements are included in this Amendment No. 1.
Except as described above, no other changes have been made to the Original Form 10-K and the Original Form 10-K continues to speak as of the Original Filing Date. Except as expressly set forth herein, this Amendment No. 1 does not reflect events occurring after the Original Filing Date or modify or update any of the other disclosures contained therein in any way other than as required to reflect the amendments discussed above. Accordingly, this Amendment No. 1 should be read in conjunction with the Original Form 10-K and our other filings made with the SEC subsequent to the filing of the Original Form 10-K. Unless the context otherwise requires, references in this Amendment No. 1 to “Axogen,” the “Company,” “we,” “our,” or “us” mean Axogen, Inc., a Minnesota corporation, and its consolidated subsidiaries.
TABLE OF CONTENTS
FORWARD-LOOKING STATEMENTS
From time to time, in reports filed with the U.S. Securities and Exchange Commission (the “SEC”) (including this Amendment No. 1), Axogen, Inc. (including Axogen, Inc.’s wholly owned subsidiaries, Axogen Corporation, Axogen Processing Corporation and Axogen Europe GmbH, the “Company,” “Axogen,” “we,” “our,” or “us”) may provide forward-looking statements, as defined in the Private Securities Litigation Reform Act of 1995, concerning possible or anticipated future results of operations or business developments. These statements are based on management's current expectations or predictions of future conditions, events, or results based on various assumptions and management's estimates of trends and economic factors in the markets in which we are active, as well as its business plans. Words such as “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “projects,” “forecasts,” “continue,” “may,” “should,” “will,” “goals,” and variations of such words and similar expressions are intended to identify such forward-looking statements.
The forward-looking statements in this Amendment No. 1 include, but are not limited to the following:
•Our expectation that we remain on track for submission of our Biological License Application (“BLA”) for Avance by the end of 2023 and anticipate final determination in 2024.
•Our belief that the BLA approval would complete the regulatory transition of Avance Nerve Graft from a 361-tissue based product, to a 351-biological product.
•Our belief that, subject to BLA approval Avance, would then be designated as a reference product, which would in turn provide 12 years of data exclusivity with regard to potential biosimilars.
The forward-looking statements are and will be subject to risks and uncertainties, which may cause actual results to differ materially from those expressed or implied in such forward-looking statements. Forward-looking statements contained in this Amendment No. 1 should be evaluated together with the many uncertainties that affect our business and its markets, particularly those discussed in the risk factors and cautionary statements set forth in our filings with the SEC, including as described in “Risk Factors” included in Item 1A of the Original Form 10-K and “Risk Factor Summary” included in the Original Form 10-K. Forward-looking statements are not guarantees of future performance, and actual results may differ materially from those projected. The forward-looking statements contained in this report are based on information that is currently available to us and expectations and assumptions that we deem reasonable at the time the statements were made. The forward-looking statements are representative only as of the date they are made and, except as required by applicable law, we assume no responsibility to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances or otherwise.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock is traded on the Nasdaq Capital Market under the symbol “AXGN.” On March 10, 2023, the last reported closing sale price of our common stock on the Nasdaq Capital Market was $7.58 per share.
Shareholders
As of March 10, 2023, we had 42,601,918 shares of common stock outstanding, and approximately 226 common shareholders of record, based upon information received from our stock transfer agent. However, this number does not include beneficial owners whose shares were held of record by nominees or broker dealers. We estimate that there are approximately 11,343 individual owners. Additional information called for by this item is incorporated herein by reference to the following sections of this Report: Note 11 - Stock-Based Compensation of the Notes to Consolidated Financial Statements included in Item 8; and Part III, Item 12 “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters – Equity Compensation Plan Information”.
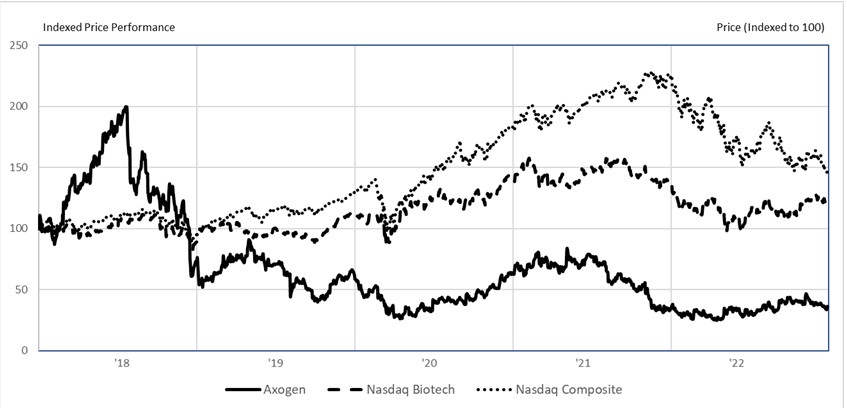
Stock Performance Graph
The following graph compares the cumulative total shareholder return on our common stock for the period from December 31, 2018 to December 31, 2022 with (i) the Nasdaq Stock Market Biotechnology Index and (ii) the Nasdaq Stock Market Composite Index. The graph assumes an investment of $100 in our common stock and the respective indices for the period of December 31, 2018 to December 31, 2022. The comparisons set forth in the graph are provided pursuant to SEC rules and are not intended to forecast or be indicative of the future performance of our common stock or either of the included indices. The performance graph shall not be deemed incorporated by reference by any general statement incorporating by reference this annual report into any filing under the Securities Act of 1933, as amended, or the Exchange Act of 1934, as amended, except to the extent we specifically incorporate this information by reference and shall not otherwise be deemed filed under such acts.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
We did not repurchase any of our securities in the fourth quarter of 2022.
Recent Sales of Unregistered Securities
We had no sales of unregistered securities in 2022.
Securities Authorized for Issuance Under Equity Compensation Plans
See Part III, Item 12 “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.”
Dividends
We have never declared or paid and do not anticipate paying or declaring a cash dividend on our common stock. We intend to retain any earnings to finance the growth and development of our business. Our Board of Directors may declare dividends at its discretion.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Information About Our Directors
Set forth below is certain information regarding the directors of the Company as of March 31, 2023. The biographies of each of the directors below contains information regarding the person’s service as a director, business experience, director positions held currently or at any time during the last 5 years, and information regarding the experiences, qualifications, attributes or skills that caused the Nominating and Corporate Governance and Committee and the Board to determine that the person should serve as a director.
| | | | | | | | | | | | | | |
| Name | | Age | | Position(s) |
| Karen Zaderej | | 61 | | | Chairman and Chief Executive Officer |
| Amy Wendell | | 62 | | | Director |
| William Burke | | 54 | | | Director |
| Gregory Freitag | | 61 | | | Director |
| John H. Johnson | | 65 | | | Director |
| Alan Levine | | 55 | | | Director |
| Guido Neels | | 74 | | | Director |
| Paul Thomas | | 67 | | | Director |
| Dr. Joseph Tyndall | | 54 | | | Director |
Karen Zaderej joined Axogen Corporation in May 2006. She has served as President, Chief Executive Officer and a Member of the Board of Directors since September 2011 and became Chairman of the Board of Directors in May 2018. She served as Chief Executive Officer and as a member of the Board of Directors of Axogen Corporation, our wholly-owned subsidiary since May 2010 and as Chief Operating Officer from October 2007 to May 2010 and as Vice President of Marketing and Sales from May 2006 to October 2007. From October 2004 to May 2006, she worked for Zaderej Medical Consulting, a consulting firm she founded, which assisted medical device companies build and execute successful commercialization plans. From 1987 to 2004, she worked at Ethicon, Inc., a Johnson & Johnson company, where she held senior positions in marketing, business development, research & development, and manufacturing. Ms. Zaderej is a member of the University of Tampa Board of Trustees and sits on the Board of Directors for EyePoint Pharmaceuticals, Inc. She has an M.B.A. from the Kellogg Graduate School of Business and a B.S. in Chemical Engineering from Purdue University. Ms. Zaderej’s qualifications to serve on our Board of Directors include her leadership and depth of knowledge of the Company, her extensive experience in the medical device industry, and her financial and management expertise.
Amy Wendell has served as a member of our Board of Directors since September 2016 and Lead Director since May 2018. She was a senior advisor for the healthcare investment banking practice of Perella Weinberg Partners (“PWP”) from January 2016 through April 2019. Her scope of responsibilities involved providing guidance and advice with respect to mergers and acquisitions and divestitures for clients and assisting PWP in connection with firm-level transactions. From 2015 through October 2018, Ms. Wendell served as a senior advisor for McKinsey and Company’s (“McKinsey”) strategy and corporate finance practice and as a member of McKinsey’s transactions advisory board to help define trends in mergers and acquisitions, as well as help shape McKinsey’s knowledge agenda. From 1986 through January 2015, Ms. Wendell held various roles of increasing responsibility at Covidien plc (“Covidien”) (including its predecessors, Tyco Healthcare and Kendall Healthcare Products), including in engineering, product management and business development. Most recently, from December 2006 until Covidien’s acquisition by Medtronic plc in January 2015, Ms. Wendell served as Covidien’s Senior Vice President of Strategy and Business Development, where she managed all business development, including acquisitions, equity investments, divestitures and licensing/distribution, and led Covidien’s strategy and portfolio management initiatives. Ms. Wendell is a member of the board of directors of Hologic, Inc., a leading developer, manufacturer and supplier of premium diagnostic products, medical imaging systems and surgical products with a strong position in women’s health and Baxter International, Inc., a leading global medical products company. She is also a director of Por Cristo, a non-profit charitable medical service organization involved in healthcare work for at-risk women and children in Latin America. Ms. Wendell has a M.S. in biomedical engineering from the University of Illinois and a B.S. in mechanical engineering from Lawrence Institute of Technology (n/k/a Lawrence Technological University). Ms. Wendell’s qualifications to serve on our Board of Directors
include her broad healthcare management and governance experience, her knowledge of healthcare policy and regulation, patient care delivery and financing, and her knowledge of clinical research and medical technology assessment.
William Burke has served as a member of our Board of Directors since July 2022. He was the Executive Vice President, Chief Financial Officer of Haemonetics Corp. (NYSE: HAE), a company that provides a suite of innovative medical technology solutions to improve the quality, effectiveness, and efficiency of care, from August 2016 through June 2022 and was responsible for the global finance organization including investor relations. From July 2014 through July 2016, Mr. Burke served as Chief Integration Officer and Vice President, Integration for Medtronic plc (NYSE: MDT), a global healthcare products company, and was a member of its executive committee. In that role, Mr. Burke was responsible for ensuring the successful integration of Medtronic with Covidien plc, a global healthcare company, following its acquisition by Medtronic. Prior to joining Medtronic, Mr. Burke spent more than 20 years in finance and business development leadership roles at Covidien, including Chief Financial Officer for Covidien Europe based in Zurich, Vice President of Corporate Strategy and Portfolio Management and Vice President of Financial Planning and Analysis. Mr. Burke also held key positions within Tyco Healthcare, including the financial controller of Valleylab, managing director of the Covidien Group in Switzerland, and international controller. Mr. Burke began his career as an auditor with KPMG. He currently serves on the board of directors of MiroMatrix (NASDAQ CM: MIRO). Mr. Burke holds a B.S. in Business Administration from Bryant College. Mr. Burke’s qualifications to serve on our Board of Directors include his management and finance experience, and his knowledge of medical technology.
Gregory Freitag has served as a member of our Board of Directors since September 2011. He was Axogen’s Special Counsel from June 2020 through his retirement in March 2021, General Counsel from September 2011 through June 2020, Chief Financial Officer from September 2011 through May 2014 and August 2015 through March 2016 and Senior Vice President Business Development from May 2014 through October 2018. Mr. Freitag was the Chief Executive Officer, Chief Financial Officer and a board member of LecTec Corporation, an intellectual property licensing and holding company that merged with Axogen in September 2011, from June 2010 through September 2011. From May 2009 to the present, Mr. Freitag has been a principal of FreiMc, LLC, a healthcare and life science consulting and advisory firm he founded that provides strategic guidance and business development advisory services. Prior to founding FreiMc, LLC, Mr. Freitag was a Director of Business Development at Pfizer Health Solutions, a former subsidiary of Pfizer, Inc., from January 2006 through May 2009. From July 2005 through January 2006, Mr. Freitag worked for Guidant Corporation in their business development group. Prior to Guidant Corporation, Mr. Freitag was the Chief Executive Officer of HTS Biosystems, a biotechnology tools start-up company, from March 2000 through its sale in early 2005. Mr. Freitag was the Chief Operating Officer, Chief Financial Officer and General Counsel of Quantech, Ltd., a public point of care diagnostic company, from December 1995 through March 2000. Prior to that time, Mr. Freitag practiced corporate law in Minneapolis, Minnesota. Mr. Freitag is also a director of PDS Biotechnology Corporation (Nasdaq: PDSB), a clinical stage biopharmaceutical company developing immunotherapies for cancer and other disease areas such as infectious disease and ZyVersa Therpeutics (Nasdaq: ZVSA), a clinical-stage specialty biopharmaceutical company focused on developing drugs to treat inflammatory and renal disease. Mr. Freitag holds a J.D. from the University of Chicago and a B.A. in Economics & Business and Law & Society from Macalester College, MN. Mr. Freitag’s qualifications to serve on our Board of Directors include his proven leadership and experience as a senior level executive, his particular knowledge of public companies, including reporting, compliance and financial markets, his finance management and legal expertise and over 30 years of experience in the life sciences sector.
John H. Johnson has served as a member of our Board of Directors since July 2021. He currently serves as the Chief Executive Officer and a Director on the Board of Reaction Biology, and has served as the Chief Executive Officer of Strongbridge Biopharma plc., a company focused on building a portfolio of vertical, therapeutically-aligned rare disease franchises, from July 2020 through October 2021, when it was sold to Xeris Biopharma Holdings, Inc. He also served as Strongbridge Biopharma's Executive Chairman from March 2015 through November 2019. Mr. Johnson previously served as the Chief Executive Officer of Melinta Therapeutics, a commercial stage company developing and commercializing novel antibiotics, from 2018 through 2020. He served as Chairman and Chief Executive Officer of Dendreon Corporation from 2012 through 2014. Mr. Johnson previously held various senior positions with Eli Lilly & Company, ImClone Systems, Inc., Johnson & Johnson, and Centocor Ortho Biotech. Mr. Johnson currently serves as non-executive chairman of the Board of Directors for Autolus Therapeutics plc, and on the Board of Directors of Xeris and Verastem Oncology. Mr. Johnson received his B.S. from University of Pennsylvania East Stroudsburg. Mr. Johnson's qualifications to serve on our Board of Directors include his considerable leadership experience and specific knowledge of the healthcare industry.
Alan Levine has served as a member of our Board of Directors since May 2019. Since February 2018, Mr. Levine has been the Chairman, President, and Chief Executive Officer of Ballad Health, an integrated healthcare delivery system. From January 2014 through January 2018, he served as the President and Chief Executive Officer of Mountain States Health Alliance, the largest health system in upper east Tennessee and southwest Virginia. He served as a Senior Advisor to the Board of Directors, President of the Florida Group and Corporate Senior Vice President during his July 2010 through January 2014
tenure at Health Management Associates, a hospital and healthcare facilities operator. From January 2008 through July 2010, Mr. Levine served as Senior Health Policy Advisor to Louisiana Governor Bobby Jindal, and as the Secretary of the Louisiana Department of Health and Hospitals on the Governor’s cabinet. He was the President and Chief Executive Officer of the North Broward Hospital District, one of the largest public health and hospital systems in the nation, from July 2006 through January 2008. He also served as the Secretary of the Florida Agency for Health Care Administration, the health planning and regulatory agency for the State of Florida with responsibility for the oversight of more than 30,000 healthcare facilities, and the $17 billion state Medicaid program, from June 2004 through July 2006. Mr. Levine served as the Deputy Chief of Staff and Senior Health Policy Advisor to Governor Jeb Bush from January 2003 through June 2004. He holds an M.B.A., M.S. in Health Science, and B.S. in Health Education/Community Health from the University of Florida. He currently serves on the Board of Governors of the State University System of Florida, where he has served as Chair of the Audit and Compliance Committee, Chair of the Research and Academic Excellence, Committee and Chair of the Select Committee on 2+2 Education Attainment. He also served as Chair of the State of Florida Higher Education Coordinating Council, a policy-setting body composed of all education entities from K-Post Secondary. Mr. Levine’s qualifications to serve on our Board of Directors include his broad healthcare management, policy and regulation and patient care delivery knowledge, executive level experience with integrated healthcare delivery systems and his knowledge as to budgeting and financial reporting.
Guido Neels has served as a member of our Board of Directors since August 2015. He has been an operating partner of EW Healthcare Partners L.P. (“EW”) since February 2013. Mr. Neels joined EW as a Partner in August 2006, was promoted to Managing Director in 2008 and served in that position until being appointed to Operating Partner. From May 2004 through his retirement in November 2005, Mr. Neels served as Chief Operating Officer of Guidant Corporation (“Guidant”), a world leader in the development of cardiovascular medical products, where he was responsible for the global operations of Guidant’s four operating units – Cardiac Rhythm Management, Vascular Intervention, Cardiac Surgery, and Endovascular Solutions. From December 2002 through May 2004, Mr. Neels was Group Chairman, Office of the President at Guidant, responsible for worldwide sales operations, corporate communications, corporate marketing, investor relations and government relations. From January 2000 through December 2002, Mr. Neels was President of Guidant for Europe, Middle East, Africa and Canada. Mr. Neels previously served as Vice President of Global Marketing for Vascular Intervention and as Managing Director for German and Central European operations. From 1982 through 1994, until Guidant was spun off as an independent public company from Eli Lilly and Co., Mr. Neels held general management, sales and marketing positions at Eli Lilly in the United States and Europe. From 1972 through 1980, he held positions in information technology, finance and manufacturing at Raychem Corporation in Belgium and the United States. Mr. Neels currently serves on the board of directors of Bioventus LLC, a portfolio company of Essex Woodlands. In addition, Mr. Neels also serves on the board of directors for Christel House International and Amici Lovanienses, both not-for-profit organizations. Mr. Neels holds an M.B.A. from Stanford University and a business engineering degree from the University of Leuven in Belgium. Mr. Neels’ qualifications to serve on our Board of Directors include his extensive leadership experience in the medical device and biotechnology industries and his expertise in the commercialization of medical devices, corporate governance and the financial markets.
Paul Thomas has served as a member of our Board of Directors since September 2020. He currently serves on the board of directors of Surgalign Spine Technologies, Inc. (NASDAQ: SRGA). Mr. Thomas has more than 30 years of experience in the medtech industry and currently serves as the CEO and Co-Founder of Prominex, Inc. He also served as the CEO of Roka Bioscience from 2009 through 2017. Prior to that, Mr. Thomas served as Chairman and CEO of LifeCell Corporation from 1998 until it was acquired by KCI in 2008 in a transaction valued at $1.8 billion. He also held various senior positions, including President of the Pharmaceutical Products Division, during his tenure of 15 years with Ohmeda, Inc. Mr. Thomas received his M.B.A. from Columbia University Graduate School of Business and completed his post graduate studies in Chemistry at the University of Georgia Graduate School of Arts and Science. He received his B.S. in Chemistry from St. Michael’s College. Mr. Thomas’ qualifications to serve on our Board of Directors include his extensive executive leadership and financial experience, particularly in connection with rapid growth technology businesses, and his experience as a director of publicly traded companies.
Dr. Joseph Tyndall has served as a member of our Board of Directors since December 2022. He is currently the Executive Vice President for Health Affairs and Professor and Dean of the Morehouse School of Medicine since July 1, 2021. Dr. Tyndall served as Professor and Chair of the Department of emergency medicine at the University of Florida College of Medicine from January 2021 through June 2021. He was appointed interim dean of the College of Medicine from August 2018 through January 2021 and was subsequently appointed to the position of Associate Vice President for Strategic and Academic Affairs for UF Health in Gainesville Florida. He served on the Board of Directors of UF Health Shands Hospital at the University of Florida from 2010 through 2021 and was chair of the Board of Trustees for the UF Health Proton Therapy Institute during his tenure as interim dean. He served on the Board of Directors of the Florida College of Emergency Physicians from 2011 through 2021 serving as the societies President from 2018 through 2019. He is currently a member of the Board of Directors of Grady Health System in Atlanta Georgia, is a trustee and President of the Society for Academic Emergency Medicine Foundation -emergency medicine’s national foundation supporting education and research in emergency care. Dr. Tyndall is a graduate of the
University of Maryland School of Medicine and the emergency medicine residency program at the University of Maryland Medical System serving as Chief Resident. He received a M.S. in Health Services Management and Health Policy from Columbia University. He is an elected member of the Alpha Omega Alpha Honor Society and the Gold Humanism Honor Society. He has published and lectured extensively nationally and internationally in emergency medicine and has active research interests in acute brain injury. He is an editor of 10th edition of the leading textbook in Emergency Medicine Rosen’s Emergency Medicine; Concepts and Clinical Practice. Dr. Tyndall's qualifications to serve on our Board of Directors include his broad healthcare management as well as clinical experience, his knowledge of healthcare policy, emergency medicine, and patient care delivery, and his knowledge of clinical research.
There are no family relationships between or among any of our directors or executive officers. The principal occupation and employment during the past 5 years of each of our directors was carried on, in each case except as specifically identified above, with a corporation or organization that is not a parent, subsidiary or other affiliate of us. There is no arrangement or understanding between any of our directors and any other person or persons pursuant to which he or she is to be selected as a director. There are no material legal proceedings to which any of our directors is a party adverse to us or any of our subsidiaries or in which any such person has a material interest adverse to us or our subsidiaries.
Information About Our Executive Officers
The names and age of all executive officers of the Company and the principal occupations and business experience for at least the last 5 years for each are set forth below as of March 31, 2023:
| | | | | | | | | | | | | | |
| Name | | Age | | Position(s) |
| Karen Zaderej | | 61 | | | Chief Executive Officer and Chairman |
| Peter Mariani | | 59 | | | Executive Vice President and Chief Financial Officer |
| Marc Began | | 56 | | | Executive Vice President, General Counsel and Chief Compliance Officer |
| Michael Donovan | | 58 | | | Vice President of Operations |
| Angelo G. Scopelianos Ph.D. | | 68 | | | Chief Research and Development Officer |
For Ms. Zaderej's biography, see "Information About Our Directors" above.
Peter Mariani has served as our Chief Financial Officer since March 2016 and was promoted to Executive Vice President and Chief Financial Officer in March of 2021. He brings more than 25 years of experience as a financial executive in private and public companies. He previously served as Chief Financial Officer of Lensar, Inc, a privately held laser refractive cataract surgery company, from July 2014 through January 2016, following the sale of Lensar in December 2015. From June 2011 through June 2014, he served as Chief Financial Officer of Hansen Medical, a publicly traded medical device company developing robotic solutions for intravascular procedures. He served as Chief Financial Officer for two privately held companies Harlan Laboratories from 2007 through 2009; and BMW Constructors from 2009 through 2010. From 1994 through 2006 he served in various senior financial roles with Guidant Corporation, a publicly traded leader in the development and sale of medical devices for the treatment of cardiovascular disease. Mr. Mariani began his career with Guidant as Director of Corporate Financial Reporting where he supported the initial IPO of Guidant and ultimately served as Vice President, Controller and Chief Accounting Officer. His experience at Guidant included two years as Director of Financial Reporting, Guidant Vascular Intervention in Santa Clara, California, and four years in Tokyo, Japan, primarily as Vice President Finance and Administration. While in Japan he helped to facilitate the conversion and scale of the Japanese business from a distributor network to a direct sales and marketing organization. Following the 2006 sale of Guidant to Boston Scientific Corporation, he co-led the initial integration of the two companies. From 1987 through 1994 Mr. Mariani worked with Ernst and Young, LLP, where he served a diverse client base as a Certified Public Accountant. Mr. Mariani earned a B.S. in Accounting from Indiana University.
Marc Began has served as our General Counsel since March of 2023. He brings more than 25 years of experience representing life science companies as in-house and external counsel. From June 2018 until its acquisition in December 2022 by Johnson and Johnson, Inc., he served as Executive Vice President, General Counsel and Secretary of Abiomed, Inc., a publicly traded med-tech company specializing in heart, lung and kidney recovery and was responsible for leading its legal, compliance and business development functions. While at Abiomed, he oversaw the acquisitions of new companies and new products. From August of 2017 until June of 2018, he was Vice President of Legal and Intellectual Property at Boehringer Ingelheim, a privately held pharmaceutical and biologics company, where he was responsible for medical device, pharmaceutical, and biologic legal and intellectual property issues. Before joining Boehringer Ingelheim, he held various positions of increasing responsibility over a 15-year period at Novo Nordisk, a publicly traded company specializing in metabolic and endocrinological diseases. Prior to becoming an in-house lawyer for Novo Nordisk, Mr. Began was in private practice at the law firms of Sullivan & Cromwell LLP and White & Case LLP in New York, where he handled a broad range of legal matters, including
litigation intellectual property, corporate transactions, regulatory and compliance for private and public companies. Mr. Began has a J.D. from Albany Law School at Union University and holds a B.S. in Mechanical Engineering from Rensselaer Polytechnic Institute.
Michael Donovan has served as our Vice President of Operations since September 2015. Prior to September 2015, Mr. Donovan was our Director of Operations from January 2011 through September 2015. From 1988 through 2010, Mr. Donovan held various positions at Zimmer Holdings in manufacturing, continuous improvement, quality assurance, and sterilization, including Director of Manufacturing from 2002 to 2010. Mr. Donovan has a B.S. degree in Chemical Engineering and an M.B.A. from the University of Akron.
Angelo Scopelianos Ph.D. has served as Axogen’s Vice President of Research and Development since September 2018 and on January 4, 2021, began serving as the Chief Research and Development Officer. From 2012 until joining Axogen, Dr. Scopelianos was an independent consultant specializing in medical devices. He began consulting after his retirement from a 24-year career at Johnson & Johnson ("J&J"). Dr. Scopelianos began at J&J in 1988 as Section Manager of R&D progressing to Manager of R&D, Director of R&D, Vice President of R&D and from October 2010 to September 2012 Senior Vice President of R&D. He joined J&J after holding research leadership positions at EI Dupont de Nemours in Wilmington, DE, and Pennwalt Corporation. Dr. Scopelianos earned a Ph.D. in Organic Chemistry from Pennsylvania State University and a Bachelor of Science from the State University of New York—Oneonta. He holds over 35 U.S. patents and numerous international patents, and his awards include the Outstanding Science Alumni Award by Pennsylvania State University; the Scientific Leadership Award in Biomaterials Science awarded by a consortium of NJ research universities.
Audit Committee and Audit Committee Financial Expert
The Audit Committee was established in accordance with section 3(a)(58)(A) of the Securities Exchange Act of 1934 as amended ("the Exchange Act"). The Audit Committee is responsible for review of audits, financial reporting and compliance, accounting and internal controls policies, healthcare compliance, information technology and cyber security, data privacy and disaster recovery capabilities and risk management programs. For audit services, the Audit Committee is responsible for the engagement and compensation of the registered independent accounting firms, oversight of their activities and evaluation of their independence. The Audit Committee has instituted procedures for receiving reports of improper record keeping, accounting or disclosure. In the opinion of the Board of Directors, each of the members of the Audit Committee has both business experience and an understanding of accounting principles generally accepted in the United States (“GAAP”) and financial statements enabling them to effectively discharge their responsibilities as members of the Audit Committee. Moreover, the Board of Directors has determined that each of Messrs. Burke and Levine and Ms. Wendell is an “audit committee financial expert” as such term is defined in Item 407(d)(5) of Regulation S-K promulgated by the U. S. Securities and Exchange Commission (the "SEC") and is an independent director. A current copy of the Company’s Audit Committee charter, which has been adopted by our Board of Directors, is posted on our website at http://ir.axogeninc.com/governance-docs. The information found on, or accessible through, our website is not incorporated into, and does not form a part of, this Amendment No. 1 or any other report or document we file with or furnish to the SEC.
Code of Business Conduct and Ethics
The Board of Directors adopted a Code of Business Conduct and Ethics, which is posted on our website https://ir.axogeninc.com/governance-docs that is applicable to all employees and directors. We will provide copies of our Code of Business Conduct and Ethics without charge upon request. To obtain a copy, please visit our website or send your written request to Investors Relations, 13631 Progress Blvd., Suite 400, Alachua, FL 32615. With respect to any amendments or waivers of this Code of Business Conduct and Ethics (to the extent applicable to our chief executive officer, principal accounting officer or controller, or persons performing similar functions) we intend to either post such amendments or waivers on our website or disclose such amendments or waivers pursuant to a Current Report on Form 8-K.
Delinquent Section 16(a) Reports
Section 16(a) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), requires our executive officers and directors, and persons who beneficially own more than 10% of our common stock to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and other equity securities of our company. Officers, directors and greater than ten percent stockholders are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file. SEC regulations require us to identify in this report anyone who filed a required report late during our most recent fiscal year.
Based on our review of forms we received or written representations from reporting persons, we believe that all reports of securities ownership and changes in such ownership required to be filed during the year ended December 31, 2022, were timely
filed, except that Mr. William Burke's initial Form 4 reporting his initial grant of stock options upon joining the board was inadvertently filed late on July 19, 2022, due to an administrative oversight.
ITEM 11. EXECUTIVE COMPENSATION.
COMPENSATION DISCUSSION AND ANALYSIS
This Compensation Discussion and Analysis (“CD&A”) provides an overview of our executive compensation philosophy, the objectives of our executive compensation program and each compensation component that we provide. In addition, we explain how and why our Compensation Committee arrived at specific compensation policies and decisions involving our named executive officers for the fiscal year ended December 31, 2022.
The following executive officers constituted our named executive officers for the fiscal year ended on December 31, 2022:
| | | | | |
| |
| Karen Zaderej | Chief Executive Officer and President |
| Peter Mariani | Executive Vice President and Chief Financial Officer |
| Michael Donovan | Vice President of Operations |
| Bradley Ottinger | General Counsel and Chief Compliance Officer |
| Angelo Scopelianos Ph.D. | Chief Research and Development Officer |
Eric Sandberg (1) | Former Chief Commercial Officer |
(1) Mr. Sandberg departed from his position as Chief Commercial Officer in July 2022.
The following discussion should be read together with the compensation tables and related disclosures set forth below.
EXECUTIVE SUMMARY
Business Highlights
We are the leading company focused specifically on the science, development and commercialization of technologies for peripheral nerve regeneration and repair. We are passionate about providing the opportunity to restore nerve function and quality of life for patients with peripheral nerve injuries. We provide innovative, clinically proven and economically effective repair solutions for surgeons and healthcare providers.
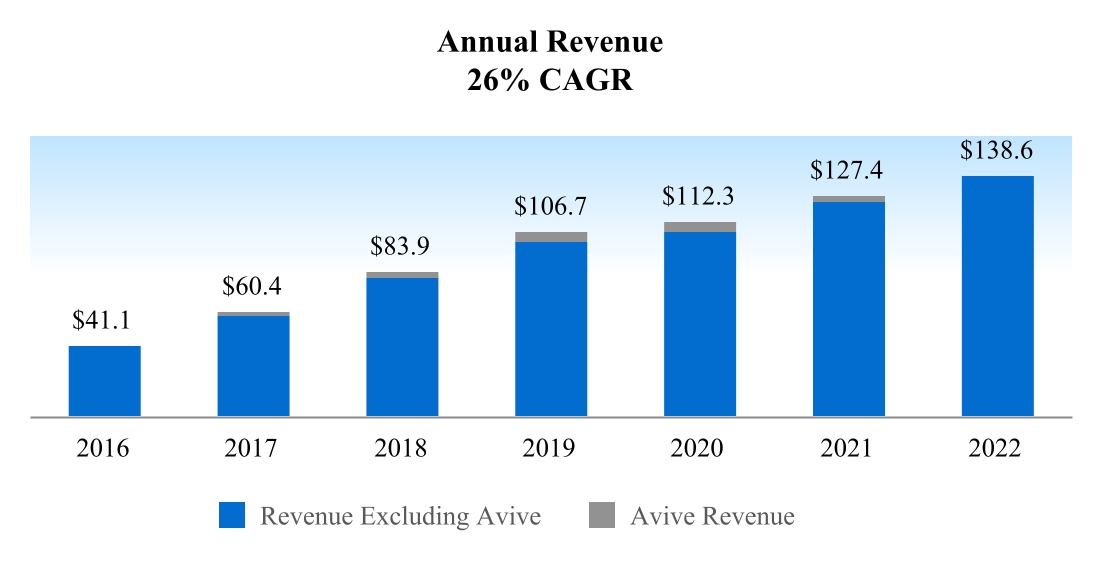
We have grown revenue rapidly and have delivered a seven-year compounded annual growth rate of 26%, while maintaining gross margins above 80%. We intend to increase market penetration and share by increasing awareness of the impact of nerve damage on quality of life and improving the adoption of nerve repair techniques and our products through the continued use of educational conferences and presentations, surgical resident and fellow training, clinical data development and publication, digital communications, and a knowledgeable and professional sales team.
Our professional education team, through surgeon training programs combined with our marketing initiatives, has been successful in developing new customers and revenue growth through increased surgeon adoption of the Axogen algorithm for nerve repair and product penetration. These efforts have included the training of approximately 75% of microsurgery fellows in both 2021 and 2022.
Although the COVID-19 pandemic and subsequent global economic challenges had a negative impact on our revenue growth, we were able to deliver revenue growth in 2021 and 2022 of 13% and 9% respectively. Revenue growth excluding the impact of Avive® Soft Tissue Membrane, sales of which were voluntarily suspended on June 1, 2021, was 15% in 2021 and 12% in 2022.
The labor shortages resulting from the COVID-19 pandemic have particularly impacted hospitals where a shortage of nursing and other staff have negatively impacted surgical schedule capacity and predictability which negatively impact nerve repair procedure. Although we believe that hospitals will continue to address these challenges and return to more normalized staffing over time, we remain measured on the pace of continued improvements as hospitals continue to work through these challenges and return to more normalized operating schedules.
We have always made clinical evidence generation an important priority and believe that our collection of meaningful data publications is the most comprehensive in the area of peripheral nerve repair. The unparalleled amount of evidence in nerve
repair is expected by our surgeons when making clinical care decisions. We have 215 peer-reviewed papers, including growing numbers across all of our nerve repair applications.
We achieved the following key clinical data milestones in 2022:
•RECON Phase 3 Study of Avance met its primary endpoint. This study provided the first ever Level 1 clinical evidence in support of Avance Nerve Graft for peripheral nerve repairs. This data will be used to support our Biologics License Application ("BLA") submission.
•Independent publication of comparative nerve gap repair meta-analysis of over 1,500 nerve repairs across 35 peer-reviewed studies of Allograft, Autograft, and Conduits concluded allograft (Avance) and autograft repairs delivered significantly better rates of meaningful sensory recovery in short gaps as compared to conduit repairs. Further, the authors concluded that there were no statistical differences between allograft and autograft outcomes in both sensory and mixed/motor nerve repairs across all gap lengths up to 70 millimeters. Additionally, the study found that acute procedure related costs were comparable between allograft and autograft procedures, in both the inpatient and outpatient settings.
We remain committed to developing the clinical evidence to demonstrate safety, performance, and utility of our nerve repair solutions to support the continued adoption of the Axogen algorithm across our full portfolio of products.
Construction of our new processing facility was completed in 2022 and the validation and transition to the new facility is in process. The new facility will support our BLA for Avance Nerve Graft and provide for our long-term processing capacity. We remain on track for submission of our BLA for Avance by the end of 2023 and anticipate final determination in 2024. A BLA approval would complete the regulatory transition of Avance Nerve Graft from a 361-tissue based product, to a 351-biological product; and, importantly, we believe Avance would then be designated as a reference product, which would in turn provide 12 years of data exclusivity with regard to potential biosimilars.
With over 75,000 Avance nerve grafts implanted since launch, we are confident that we have built the right organization with a solid foundation of clinical evidence that will allow us to continue to lead and innovate in this large and developing peripheral nerve repair market.
Executing our Strategy
We have made great progress toward our commercial strategy of driving strong surgeon adoption of our technology. Some highlights of our progress include:
a.Revenue CAGR of 26% over past 7 years
b.Gross margins > 80%
c.Ended 2022 with 115 direct sales representatives.
d.Ended 2022 with 215 peer-reviewed clinical publications featuring Axogen’s nerve repair products
e.More than 75,000 Avance® Nerve Grafts have been implanted since launch
Continued Revenue Growth
2022 was another year of strong revenue growth, with an increase of approximately 12% from 2021, excluding the impact of Avive Soft Tissue Membrane revenue in 2021.
Executive Compensation Philosophy
As our Company has continued to evolve with rapid growth and clinical success, it has been imperative that the Compensation Committee continually evaluate and transform the executive compensation program to appropriately structure pay packages in consideration of the company's size, investor expectations, and industry standards. Our Compensation Committee firmly believes that executive compensation should be linked to our overall performance with particular focus on driving long-term, sustainable revenue growth. As such, our executive compensation program is designed to attract highly qualified individuals, retain those individuals in a competitive marketplace and motivate performance in a manner that supports achievement of our corporate goals while ensuring that these programs do not encourage excessive risk-taking. We believe our executive compensation program, as presented in this CD&A, achieves these objectives.
Key Compensation Decisions and Outcomes in 2022
In 2022, the Compensation Committee and the Company continued to undertake reviews of the appropriateness of key elements of executive compensation and continued to grant a mix of performance share units ("PSUs"), restricted share units ("RSUs") and stock options in the first quarter of the year.
In March of 2022, the Compensation Committee approved a provision to our equity awards to those employees with a title of Vice President and above. This provision generally provides for continued vesting of equity awards after a specific event which is generally defined as the termination of service after attainment of age sixty (60) with at least ten (10) years of continuous service, provided that the employee has provided at least twelve (12) months of advance notice of the retirement date for Executives, or 6 months of advance notice for Vice Presidents.
Also, in March of 2022 PSUs were granted with performance metrics based on revenue growth using 2021 as the baseline with three (3) annual measurement periods beginning in 2022 through 2024 (see discussion of specific targets below).
Additionally, the Compensation Committee determined that the stock option awards provided to Ms. Zaderej, Mr. Mariani, and Mr. Sandberg on March 16, 2022, would contain a premium pricing provision whereby the exercise price of the award was set 25% above the closing stock price on the date of grant.
During 2022, the Compensation Committee determined that the revenue growth targets for 2022 set for the PSU awards granted in 2021, were not likely to be achieved and would be forfeited by the executives upon completion of their respective performance periods. Notwithstanding this determination, the Compensation Committee made no adjustments to this program.
In February 2022, consistent with evolving practices regarding Board and Executive stock ownership guidelines, the Board of Directors amended the forms of equity interests included in the guidelines to exclude the "In-The-Money" value of vested
stock option awards and include the value of unvested Restricted Stock Units. Unearned performance stock units remain excluded from the policy. Based upon the significant change in the policy, Executives and Board Members were provided a new 5-year period to achieve compliance.
Say on Pay Vote
The Compensation Committee considered the results of our non-binding advisory vote on executive compensation (commonly referred to as a “say-on-pay” vote) at our 2022 annual meeting of stockholders in its determination of the Company’s compensation programs and policies for our named executive officers. The results of our engagement with our shareholders after our 2022 annual meeting will be discussed in greater detail in the proxy statement to be filed for our 2023 annual meeting of shareholders.
Pay Program Overview
We believe that the design and structure of our pay program, and in particular our incentive plans, support our business strategy and organizational objectives while successfully aligning executive focus and interest with that of shareholders. Our compensation programs are designed to attract, motivate and retain qualified and talented executives, motivating them to achieve our business goals and rewarding them for superior short-term and long-term performance. All pay elements, and the safeguards and governance features of the program, have been carefully chosen and implemented to align with our pay philosophy and objectives.
In doing so, we have selected the following framework to achieve these objectives:
| | | | | |
| |
Base Salary | Base salaries are set to be competitive within our industry and are important in attracting and retaining talented executives. Base salaries are fixed pay set with consideration for responsibilities, market data and individual contribution. |
Annual Cash Incentives | The annual cash incentive award plan is intended to motivate and reward our executives for the achievement of certain strategic goals of the Company. In 2022, our annual incentives were based on key corporate objectives, including revenue and certain other operational goals. |
Long-Term Equity Incentives | Long-term equity awards incentivize executives to deliver long-term shareholder value, while also providing a retention vehicle for our top executive talent. Equity awards are typically delivered as: a.PSUs b.RSUs c.Stock options |
Compensation Governance
Our Compensation Committee is responsible for oversight of the Company’s compensation program and practices. A significant part of this responsibility is aligning the Company's compensation program with the Company’s business strategies and goals, as well as the interest of our shareholders, while also mitigating excessive risk taking. To that end, the Company has committed to numerous governance practices and safeguards to ensure the compensation program does not misalign those interests.
| | | | | |
| |
What We Do | |
✔ Pay-for-performance philosophy and culture | ✔ Engage an independent compensation consultant |
✔ Provide an appropriate mix of performance-based and time-vesting awards to executives | ✔ Appropriate stock ownership requirements for all executives and non-executive directors |
✔ Strong emphasis on performance-based incentive awards | ✔ Perform an annual risk assessment of our compensation program |
✔ Responsible use of shares under our long-term incentive program | ✔ Annual say on pay vote |
What We Don’t Do | |
X Hedging or pledging of Company securities | X Excessive perquisites |
X Excise tax gross-ups | X Backdating or repricing of stock option awards |
| X No single trigger change of control cash payments | X No guaranteed minimum bonuses or uncapped incentives under our annual cash incentive plan |
| X No non-qualified defined contribution or other deferred compensation plan | X No changes to PSU targets subsequent to grant date |
X Have not changed annual incentive bonus plan targets, other than 2020 COVID-related changes. | |
EXECUTIVE COMPENSATION PHILOSOPHY AND OBJECTIVES
Our compensation philosophy is designed to pay for performance and achieve the following principal objectives:
•align our executive officers’ compensation with our business objectives and the interests of our shareholders;
•enable us to attract, motivate and retain the level of successful, qualified senior executive leadership talent necessary to achieve our long-term goals; and
•reward performance, company growth and advancement of our long-term strategic initiatives.
We carefully construct pay packages to appropriately balance fixed and variable elements to achieve the aforementioned objectives.
COMPENSATION-SETTING PROCESS
Role of Compensation Committee
Our Compensation Committee is responsible for, among other things, overseeing our executive compensation philosophy and our executive compensation program, determining and approving the compensation for our named executive officers, negotiating executive employment contracts, and helping to establish appropriate compensation for directors and other key employees. Our Compensation Committee regularly reports to our Board of Directors on its deliberations, but is ultimately responsible for compensation decisions, as described in the Compensation Committee’s Charter.
Our Compensation Committee reviews, on at least an annual basis, our executive compensation program, including our incentive compensation plans, to determine whether they are appropriate, properly coordinated, and achieve their intended purposes, and recommends to our Board of Directors any modifications or new plans or programs. It also reviews the compensation of our named executive officers and makes decisions about the various components that comprise their compensation packages.
Role of Consultants
Since May 2016, our Compensation Committee has engaged Aon's Human Capital Solutions practice, a division of Aon plc ("Aon"), to provide the Compensation Committee with a thorough analysis of our executive compensation, focusing on all compensation components. In 2022, Aon assisted the Compensation Committee with, among other things:
•Executive and director market pay analysis;
•Reviewing and modifying the compensation peer group;
•Development of 2022 executive and director pay programs; and
•Reviewing our Compensation, Discussion & Analysis disclosure.
The Compensation Committee annually evaluates the independent compensation consultant’s independence and performance under the applicable SEC and Nasdaq listing standards. The Compensation Committee believes that working with an independent compensation consultant furthers the Company’s objectives to recruit and retain qualified executives, align their interests with those of shareholders and ensure that their compensation packages will appropriately motivate and reward ongoing achievement of business goals. The Compensation Committee conducted a specific review of its relationship with Aon in 2022 and determined that Aon’s work for the Compensation Committee did not raise any conflicts of interest.
Role of Management
The Company’s Chief Executive Officer (“CEO”), Chief Financial Officer (“CFO”), Chief Human Resources Officer (“CHRO”) and General Counsel ("GC") are involved in the design and implementation of our executive compensation and are typically present at Compensation Committee meetings, except that the CEO, CFO, CHRO and GC are not present during any voting or deliberations on their salary and equity compensation. In 2022, the CEO, CFO and CHRO reviewed the analysis and recommendations of Aon with the Compensation Committee and made recommendations regarding proposed salary, equity awards and bonus for our officers (other than themselves). The Compensation Committee exercises its discretion in accepting, rejecting and/or modifying any such executive compensation recommendations and approves all compensation and equity awards.
Use of Competitive Data
To assess the competitiveness of our executive compensation program and compensation levels, our Compensation Committee, with the assistance of Aon, examines the competitive compensation data for senior executives of our peer companies.
The Compensation Committee uses the peer group to reference recent market data and understand the marketplace. However, the Committee also recognizes the importance of flexibility and considers other factors as well, such as individual performance, experience, internal equity, history and scope of responsibility, current market conditions and the specific needs of the business at critical points in time.
2022 Peer Group
For our 2022 Peer Group, Compensation Committee conducted its regular review of companies similar to us with respect to sector and market capitalization, as well as revenue and headcount, to provide a broad perspective on competitive pay levels and practices.
•Sector – HealthCare Equipment & Supplies companies; also considered biotech/biopharma companies to broaden our market perspective
•Market Capitalization – 1/3x to 4x Axogen’s market capitalization
•Revenue – 1/3x to 4x Axogen’s projected revenues
•Headcount – 1/3x to 4x Axogen’s projected headcount
In September 2021, the Compensation Committee, with the assistance of Aon, evaluated the appropriateness of the continued inclusion of each company in our 2022 peer group. Using the criteria listed above, our Compensation Committee removed five companies from the peer group: Natera, Neogen, Nevro, NovoCure and Repligen, and added the following five companies: Bioventus, Cutera, SeaSpine, Treace Medical Concepts and Zynex for a total of 18 companies that comprise the Company's 2022 Peer Group:
| | | | | | | | |
| Alphatec | Flexion Therapeutics | SI-Bone |
| AtriCure | Glaukos | STAAR Surgical |
| Bioventus | Intersect ENT | Tactile Systems Technology |
| Cardiovascular Systems | iRhythm Technologies | Treace Medical Concepts |
| CryoLife | Luminex | Vericel |
| Cutera | SeaSpine | Zynex |
Consideration of Compensation Risk
Our pay-for-performance philosophy and compensation governance practices provide an appropriate framework to our executives to achieve our strategic goals without encouraging them to take excessive risks in their business decisions.
On an annual basis, the Compensation Committee conducts a thorough risk assessment of the Company’s compensation programs and practices to analyze whether they encourage employees to take excessive or inappropriate risks. To help with this assessment, Aon provides a detailed review of the Company’s compensation program and associated risks. The assessment focuses on the following areas of the Company’s practices and policies:
•Total direct compensation and benchmarking (level of pay and approach to setting pay)
•Annual incentive plan risk
•Equity plan risk
•Change-in-control policies
•Investor risk and other policies
After completing this review, the Compensation Committee concluded the Company’s compensation programs are, on balance, consistent with market practice and do not present excessive or inappropriate risks to the Company.
EXECUTIVE COMPENSATION PROGRAM COMPONENTS
The key elements of our executive compensation packages are base salary, annual cash incentives, and long-term equity-based awards. Our Compensation Committee believes that a combination of these elements offers the best approach to achieving our compensation goals, including attracting and retaining talented executives and motivating our executives and other officers to expend maximum effort to achieve our strategic business goals, including the creation of long-term, sustainable growth of shareholder value.
The following describes each component of our executive compensation program, the rationale for each component and how compensation amounts, and awards were determined for 2022 compensation.
Base Salaries
Base salary represents the fixed portion of our named executive officers’ compensation, which we view as an important element to attract, retain and motivate highly talented executives by rewarding the individual value that each executive officer brings to us through experience and past and expected future contributions to our success.
The Compensation Committee annually reviews the base salaries of our executive team with input from our CEO, CFO and CHRO (other than with respect to their own base salary). In addition to this input, for each executive the Compensation Committee considers:
•The individual’s role and responsibilities;
•Individual contribution and performance of the past year;
•Overall experience and expertise;
•Prior base salary;
•Corporate performance; and
•Salaries for similar positions within our industry.
Base salaries were adjusted as follows for our named executive officers in 2022: | | | | | | | | | | | |
| | | |
| Executive | 2021 Base Salary ($) | 2022 Base Salary ($) | % Change |
| Karen Zaderej | 651,200 | | 673,992 | | 3.5 | % |
| Peter Mariani | 443,080 | | 457,259 | | 3.2 | % |
| Michael Donovan | 336,735 | | 349,194 | | 3.7 | % |
| Bradley Ottinger | 376,000 | | 389,534 | | 3.6 | % |
| Angelo Scopelianos Ph.D. | 395,000 | | 406,850 | | 3.0 | % |
Eric Sandberg (1) | 364,000 | | 367,640 | | 1.0 | % |
(1) Mr. Sandberg departed from his position as Chief Commercial Officer in July 2022.
2022 Annual Cash Incentive
We provide our executives, including the named executive officers, with the opportunity to annually earn cash incentives to encourage the achievement of corporate and individual objectives and to reward those individuals who significantly impact our corporate results.
In February 2022, our Compensation Committee approved, and our Board ratified, our performance-based bonus award plan for 2022 (the “2022 Annual Cash Incentive”). Under this plan, each named executive officer was eligible to receive an annual cash bonus based on the extent to which Axogen achieved certain key corporate objectives during the 2022 fiscal year relating to revenue and significant operational goals. We provided the parameters and certain details associated with the operational goals in the footnotes to the below table. However, due to their strategic significance, we are not disclosing the exact details of the operational goals as such detailed disclosure would cause future competitive harm to the company and may inform our competitors of the trends within our business to our detriment.
Total bonus payouts were capped at 200% of target.
The Compensation Committee approved these performance goals for the 2022 Annual Cash Incentive because, in its view, they were closely linked to the successful execution of our annual operating plan and because achieving the target level of success in the corporate objectives would require a focused and consistent effort by our executive officers throughout the 2022 fiscal year.
Individual bonuses paid, if any, are calculated by multiplying the executive’s annual base salary, target bonus percentage, and percentage achievement of the corporate goals, which may be measured by reference to pre-established goals with respect to the metrics, weighting and the ultimate achievement, as summarized below.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Metrics | Minimum threshold | Minimum % of Target Bonus | Target threshold | Target % of Bonus | Maximum threshold | Maximum % of Target Bonus | Performance Achieved | Earned % of Target Bonus |
Revenues (1) | $140M; 14% growth | 35% | $150M; 22% growth | 70% | $167M 35% growth | 140% | $138.6M; 12.4% growth | —% |
| Certain Clinical Data Milestone Target Achievement | (2) | 7.5% | (2) | 15% | (2) | 30% | (2) | 14% |
| A Specific New Product Innovation Milestone Achievement | (3) | 5% | (3) | 10% | (3) | 20% | (3) | —% |
| Product and Quality Systems Management Goal Achievement | (4) | 2.5% | (4) | 5% | (4) | 10% | (4) | 10% |
| Total | | 50% | | 100% | | 200% | | 24.0% |
(1) In the first half of 2022, the world was still recovering from the impact of COVID-19 along with an increasingly negative global economic environment. The related labor shortages had a particularly negative impact on hospitals where a shortage of nursing and other staff negatively impacted surgical schedule capacity and predictability, both of which negatively impacted nerve repair procedure volumes. This resulted in lower than expected revenue growth of approximately 8% in the first half of the year, compared to our annual plan growth of 22%. Although we were pleased with the improvement of revenue growth in the second half of the year to approximately 17%, that performance was not sufficient to offset the short-fall of the first half of 2022. The Company, therefore, did not meet the minimum annual revenue threshold established by the Compensation Committee at the beginning of the year and the net payout earned for this goal was 0% out of a target of 70%.
(2) The clinical data goal included milestones related to the timing and content for the reporting of the topline data of our RECON study, the completion of the clinical study report, and other related content for our submission of the BLA for Avance Nerve Graft. We achieved the majority of these milestones as planned, including the read-out of the top-line data for RECON on May 4, 2022. The net payout earned for this goal was 14% out of a target of 15%.
(3) In May 2022, management decided to defer the specific product innovation program to 2023. This decision was made in response to the revenue shortfall to our plan in the first half of 2022 as part of a broader plan to manage spending for 2022. As a result of the deferral of the product innovation targets, this milestone was not achieved. The net payout earned for this goal was 0% out of a target of 10%.
(4) Our product and quality management system is a key priority and is regularly reviewed by the FDA, and other regulatory bodies through a series of audits of our various operating sites. These agencies can provide a series of comments as part of their audits that range from minor observations of potential improvements, to more serious observations of major non-compliance and formal warning letters. Our product and quality system management goal established a minimum threshold that would not payout if we received a major observation, and allowed for a maximum payout if no observations were received in connection with any regulatory audits. In 2022 the Company did not receive any observations and paid the maximum payout of 10% of target on a goal of 5%.
For our named executive officers, a total of 24% of targeted bonus amounts were earned in 2022. Annual cash incentives earned were as follows: | | | | | | | | | | | | | | | | | |
| Executive | Salary ($) | Target Bonus % | Target Bonus 2022 ($) | 2022 Actual Bonus Paid ($) | |
| Karen Zaderej | 673,992 | 100% | 673,992 | 161,998 | |
| Peter Mariani | 457,259 | 55% | 251,492 | 60,438 | |
| Michael Donovan | 349,194 | 50% | 174,597 | 42,022 | |
| Bradley Ottinger | 389,534 | 50% | 194,767 | 46,792 | |
| Angelo Scopelianos, Ph.D. | 406,850 | 50% | 203,425 | 48,878 | |
Eric Sandberg (1) | 367,640 | 50% | 183,820 | — | |
(1) Mr. Sandberg's employment with the Company ended in July 2022 and, therefore, did not receive a bonus payment.
Equity Compensation
We use equity awards to motivate and reward our named executive officers, to encourage long-term corporate performance based on the value of our common stock and to align the interests of our named executive officers with those of our shareholders. We firmly believe that a large percentage of an executive’s compensation package should be at-risk and linked to performance.
Since 2020, we have evaluated performance in the first quarter of each year to allow for completion of the calendar year and give us the ability to review full-year performance.
We typically utilize the following mix of equity awards as the long-term incentive component of their compensation packages:
•Performance share units (“PSUs”)
◦PSUs are granted subject to achievement of certain performance milestones, which are generally measured over multiple years, and vest subject to specific terms as documented in the PSU agreement, and approved by the Compensation Committee.
•Restricted share units (“RSUs”)
◦Vesting occurs over 4 years from the date of grant, typically with 50% vesting after 24 months and an additional 25% vesting on the third and fourth anniversaries of the grant date.
•Stock options
◦All shares underlying the options will be fully vested four years from the option grant date, with 50% of the aggregate shares vesting 24 months from the option grant date and an additional 12.5% of aggregate shares vest every six months thereafter
2022 Equity Grants
Our Compensation Committee strives to balance various long-term incentive vehicles to provide an appropriate balance of performance-based and time-vesting awards. On March 8, 2022, the Compensation Committee approved, and the Board of Directors ratified, our annual equity award grants to our named executive officers as part of their 2022 pay packages.Consistent with historical practice, the award to our CEO was greater than 50% performance-based when considering the PSUs granted and options granted at a 25% premium exercise price (for the 2022 award) to assure strong alignment with long term operational goals and stock price performance.
The 2022 equity grants consisted of:
| | | | | | | | | | | |
| Executive | PSUs (#) | RSUs (#) | Stock Options (#) |
| Karen Zaderej | 89,467 | 169,694 | 317,185 |
Peter Mariani | 60,000 | 76,780 | 103,079 |
| Michael Donovan | 25,000 | 42,563 | 39,700 |
| Bradley Ottinger | 25,000 | 44,973 | 41,948 |
| Angelo Scopelianos Ph.D. | 25,000 | 37,250 | 34,745 |
Eric Sandberg (1) | 40,000 | 62,910 | 60,576 |
(1) Upon Mr. Sandberg's separation from the Company in July 2022, these awards were forfeited. |
Achievement of PSU Grants
On December 18, 2017, December 27, 2018, and December 17, 2019, the Compensation Committee approved PSU awards to certain employees, including Dr. Scopelianos and Mr. Donovan from our NEO group related to their work on the BLA. As of December 31, 2022, 294,968 PSU awards have been granted. The number of shares available for grant is linked to certain milestones related to the BLA submission to and approval by the FDA. These awards are expected to vest beginning when the BLA is submitted to the FDA. The performance measure is based upon achieving each of the specific milestones and will vest 50% upon achievement each of the milestones and 50% one year later.
In March 2020, the Compensation Committee of the Board of Directors approved PSU awards of 363,000 shares with the performance metrics tied to 2021 revenue with a payout ranging from 0% to 200% upon achievement of specific revenue goals. In first quarter of 2022, it was determined that the performance metric was not achieved and the awards were forfeited.
On July 17, 2020, the Compensation Committee approved PSU awards of 144,300 shares with a performance metric tied to 2020 revenue with a payout ranging from 0% to 200% upon achievement of specific revenue goals. These awards were granted in mid-year with certain revenue targets adjusted for the impact of COVID-19. In the first quarter of 2021, it was determined that these awards had reached 110% of their targeted performance as reported revenue at December 31, 2020 was $112.3 million.
On March 8, 2021, the Compensation Committee approved PSU awards of 332,200 shares tied to 2022 revenue targets, with a payout ranging from 0% to 200% upon achievement of specific revenue goals. In the first quarter of 2023, it was determined that the performance metric for these awards was not achieved, and the awards were forfeited.
On March 10, 2022, the Compensation Committee approved PSU awards of 526,467 shares tied to three-year revenue achievement metrics across 2022 through 2024 with a pay-out range from 0% to 150% upon achievement of specific annual revenue growth targets of between 10% and 25%, with a 100% of target payout set at 15% annual revenue growth, excluding the impact of Avive revenue in 2021 on the 2022 annual revenue growth target. The program is measured on two separate calculations and the final payment will be the greater of the following:
•One third of the targeted award based upon achievement of annual revenue growth achievement in each of the three- year measurement periods, and
•The compounded annual growth rate (CAGR) across a three-year period, where the minimum threshold of the three-year CAGR is increased to 12.5%
The award will be earned and vest upon the final determination of the annual revenue growth in 2024 and the CAGR across the three year period. In the first quarter of 2023, it was determined that the Company had achieved the 2022 annual revenue growth of 12.4% against the target of 15%, therefore, 74% of the first-year target was achieved.
ADDITIONAL COMPENSATION PRACTICES AND POLICIES
Executive Stock Ownership Guidelines
The Board of Directors has adopted stock ownership guidelines for our executive officers. Under these guidelines, the Chief Executive Officer and each other individual serving as an executive officer must hold a specified dollar value of Axogen’s common stock and the value of unvested RSU's.
| | | | | |
| Position | Requirement |
Chief Executive Officer | 3x base salary |
Executive Officers other than CEO | 1x base salary |
All other Section 16(b) Reporting Officers | 1x base salary |
For the purposes of determining stock ownership levels, the following forms of equity interests are included: shares owned by the executive officer directly or held in trust for the benefit of the executive officer or his or her immediate family members residing in same household or through trusts; and the value of unvested RSUs. The applicable guidelines must be met within the earliest of five years from: (i) joining the Company, (ii) promotion to an officer level (iii) establishment of the guidelines or (iv) five years from February 2022 when our Board of Directors amended the guidelines. Each of our executive officers, as of December 31, 2022, are in compliance with the policy.
Anti-Hedging and Pledging Policies
All of our executive officers and members of our Board of Directors are prohibited from entering into hedging or pledging transactions in respect of our common stock or other securities issued by Axogen.
Compensation Recovery Policy
We have not implemented a policy regarding retroactive adjustments to any cash or equity-based incentive compensation paid to our named executive officers and other employees where the payments were predicated upon the achievement of financial results that were subsequently the subject of a financial restatement. In light of recent rule making, we will implement such a policy once the final NASDAQ compliance rules are in place.
Retirement and Other Benefits
Our named executive officers are eligible to participate in our tax-qualified Section 401(k) retirement savings plan on the same basis as our other employees. Employees are eligible to participate in the 401(k) plan immediately upon commencing employment, and enrollment is available any time during employment. Participating employees may make annual pretax contributions to their accounts up to a maximum amount as limited by law. The 401(k) plan requires us to make matching contributions of between 3% and 4% of the employee’s annual salary as long as the employee participates in the 401(k) plan. Both employee contributions and our contributions are fully vested at all times. In 2022, our matching contribution was 3% for the first 3% of compensation contributed and 1% for the next 2% of compensation contributed of each named executive officer’s annual base salary. We contributed, on an aggregate basis, approximately $70,000 in matching funds for our named executive officers in 2022.
Additional benefits received by our named executive officers include medical, dental, vision, short-term disability, long term disability, life and accidental death and dismemberment insurance. These benefits are provided on substantially the same basis as to all our full-time employees.
Historically, we have not provided perquisites or other personal benefits to our named executive officers. We do not view perquisites or other personal benefits as a component of our executive compensation program. Our future practices with respect to perquisites or other personal benefits will be approved and subject to periodic review by our Compensation Committee.
As noted above, in March of 2022, the Compensation Committee approved a provision to our equity awards to those employees with a title of Vice President and above. This provision generally provides for continued vesting of equity awards after a specified event which is generally defined as the termination of service after attainment of age sixty (60) with at least ten (10) years of continuous service, provided that the employee has provided at least twelve (12) months of advance notice of the retirement date for Executives, or six (6) months of advance notice for Vice Presidents.
Post-Employment Compensation Arrangements
The employment agreements provide each of our named executive officers with certain protection in the event of his or her termination of employment under specified circumstances, including termination of employment following a change in control of our Company (i.e. double trigger payment). We believe that these protections serve our executive retention objectives by helping our named executive officers maintain continued focus and dedication to their responsibilities to maximize shareholder value, including in the event that there is a potential transaction that could involve a Change in Control of our Company. The terms of these agreements were determined after review by our Compensation Committee of our retention goals for each named executive officer and an analysis of competitive market data.
For a summary of the material terms and conditions of these severance and Change in Control arrangements, see the section entitled “Executive Compensation — Potential Payments Upon Termination or Change in Control.”
Tax and Accounting Considerations
Taxation of “Parachute” Payments and Deferred Compensation
Sections 280G and 4999 of the Internal Revenue Code provide that named executive officers and directors who hold significant equity interests and certain other service providers may be subject to an excise tax if they receive payments or benefits in connection with a Change in Control of our Company that exceed certain prescribed limits, and that we (or a successor) may forfeit a deduction on the amounts subject to this additional tax. Section 409A of the Internal Revenue Code imposes significant additional taxes in the event that an employee, including a named executive officer, director, or service provider receives “nonqualified deferred compensation” that does not satisfy the conditions of Section 409A.
We did not provide any named executive officer with a “gross-up” or other reimbursement payment for any tax liability that he or she might owe as a result of the application of Sections 280G, 4999 or 409A of the Internal Revenue Code during 2022. We have not agreed and are not otherwise obligated to provide any named executive officer with a “gross-up” or other reimbursement under these sections.
Accounting for Stock-Based Compensation
We follow the FASB ASC Topic 718 for our stock-based compensation awards. ASC 718 requires companies to calculate the grant date “fair value” of their stock-based awards using a variety of assumptions. This calculation is performed for accounting purposes and reported in the compensation tables that accompany this Compensation Discussion and Analysis, even though recipients may never realize any value from their awards. ASC 718 also requires companies to recognize the compensation cost of their stock-based awards in their statements of operations over the period that the recipient of the award is required to render service in exchange for the award.
COMPENSATION COMMITTEE REPORT
The Compensation Committee has reviewed and discussed with management the Compensation Discussion and Analysis required by Securities and Exchange Commission regulations. Based on its review and discussions, the Compensation Committee recommended to the Board that the Compensation Discussion and Analysis be included in this Amendment No. 1 to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022.
Submitted by:
The Compensation Committee of the Board of Directors
Paul Thomas (Chairman)
Alan Levine
Guido Neels
Summary Compensation Table
The following table sets forth the compensation for the fiscal years 2022, 2021 and 2020 for our named executive officers.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| Name and Principal Position | | Year | | Salary($)(1) | | Bonus ($) | | Stock Awards($)(2) | | Option Awards($)(3) | | Non-Equity Incentive Plan Compensation ($)(4) | | All Other Compensation ($)(5) | | Total ($) |
Karen Zaderej (6) | | 2022 | | 673,992 | | | — | | | 2,125,120 | | | 1,420,989 | | | 161,998 | | | 16,193 | | | 4,398,292 | |
| President, CEO | | 2021 | | 651,200 | | | — | | | 3,356,055 | | | 847,962 | | | 499,347 | | | 6,011 | | | 5,360,575 | |
| | 2020 | | 560,835 | | | — | | | 2,161,653 | |
| 351,276 | | | 966,971 | | | 10,747 | | | 4,051,482 | |
Peter Mariani (6) | | 2022 | | 457,259 | | | — | | | 1,121,596 | | | 461,794 | | | 60,438 | | | 14,801 | | | 2,115,888 | |
| Executive Vice President , CFO | | 2021 | | 438,257 | | | — | | | 1,275,510 | | | 675,270 | | | 186,850 | | | 71,296 | | | 2,647,183 | |
| 2020 | | 361,800 | | | — | | | 502,623 | | | 188,623 | | | 434,032 | | | — | | | 1,487,078 | |
Michael Donovan (7) | | 2022 | | 349,194 | | | — | | | 510,450 | | | 159,132 | | | 42,022 | | | 14,802 | | | 1,075,600 | |
| Vice President of Operations | | 2021 | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| 2020 | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Bradley Ottinger | | 2022 | | 389,534 | | | — | | | 573,779 | | | 192,122 | | | 46,792 | | | 13,592 | | | 1,215,819 | |
| General Counsel and Chief Compliance Officer | | 2021 | | 375,998 | | | — | | | 654,274 | | | 260,145 | | | 144,164 | | | 88,509 | | | 1,523,090 | |
| 2020 | | 332,550 | | | 25,000 | | | 442,260 | | | 318,920 | | | 150,244 | | | 35,852 | | | 1,304,826 | |
| Angelo Scopelianos, Ph.D | | 2022 | | 406,850 | | | — | | | 554,017 | | | 181,826 | | | 48,878 | | | 85,184 | | | 1,267,797 | |
| Chief Research and | | 2021 | | 394,659 | | | — | | | 591,753 | | | 376,380 | | | 151,422 | | | 67,913 | | | 1,582,127 | |
| Development Officer | | 2020 | | 332,550 | | | — | | | 398,265 | | | 105,120 | | | 286,685 | | | 76,259 | | | 1,198,879 | |
Eric Sandberg (6)(8) | | 2022 | | 214,928 | | | — | | | 843,862 | | | 271,380 | | | — | | | 581,901 | | | 1,912,071 | |
| Former Chief Commercial | | 2021 | | 364,000 | | | — | | | 740,214 | | | 260,145 | | | 153,400 | | | 11,600 | | | 1,529,359 | |
| Officer | | 2020 | | 315,000 | | | — | | | 482,273 | | | 105,120 | | | 298,000 | | | 92,964 | | | 1,293,357 | |
(1)Salary amounts for 2020 reflect amounts actually received by the NEOs after giving effect to the 20% reduction in base salaries that became effective on April 27, 2020, and expired on October 25, 2020, as a result of cost mitigation initiatives put in place by the Company during COVID-19.
(2)Stock award amounts are calculated based on the aggregate grant date fair value computed in accordance with ASC Topic 718 as of December 31, 2022. For information regarding assumptions underlying the valuation of such, see Note 11 of the Consolidated Financial Statements in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed on March 14, 2023.
(3)Option award amounts are calculated based on the aggregate grant date fair value computed in accordance with ASC Topic 718 as of December 31, 2022. For information regarding assumptions underlying the valuation of such, see Note 11 of the Consolidated Financial Statements in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed on March 14, 2023.
(4)Bonuses were earned in the respective year and paid in the following year after final Compensation Committee approval.
(5)All other compensation, as summarized in the following table for 2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name | | 401(k) Company Match ($) | | Life Insurance Premiums ($) | | Relocation and Related Tax Assistance ($) | | Severance ($) (8) | | Total ($) |
Karen. Zaderej | | 12,200 | | | 3,993 | | | — | | | — | | | 16,193 | |
Peter Mariani | | 12,200 | | | 2,601 | | | — | | | — | | | 14,801 | |
Michael Donovan | | 12,200 | | | 2,602 | | | — | | | — | | | 14,802 | |
| Bradley Ottinger | | 12,200 | | | 1,392 | | | — | | | — | | | 13,592 | |
| Angelo Scopelianos, Ph.D. | | 12,200 | | | 7,984 | | | 65,000 | | | — | | | 85,184 | |
| Eric Sandberg | | 8,484 | | | 1,452 | | | — | | | 571,965 | | | 581,901 | |
(6) Stock options issued on March 16, 2022, to Ms. Zaderej, Mr. Mariani and Mr. Sandberg were premium-priced with an exercise price 25% greater than the closing market price on the date of grant.
(7) Mr. Donovan qualified as a NEO for 2022, however, he did not qualify as an NEO for either 2021 or 2020; therefore, no compensation information has been provided for those years.
(8) Severance includes the following: severance of $521,040, accrued vacation of $28,280 and cobra payments for 12 months of $22,645.
Grants of Plan-Based Awards
The following table provides information regarding plan-based awards granted to our named executive officers in 2022: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | Grant Date | Estimated Future Payouts Under Non-Equity Incentive Plan Awards | | Estimated Future Payouts Under Equity Incentive Plan Awards | All Other Stock Awards: Number of Shares of Stock or Units | Exercise or Base Price of Option Awards ($/Sh) | Grant Date Fair Value of Stock and Option Awards ($)(1) |
| Threshold ($) | Target ($) | Maximum ($) | | Threshold (#) | Target (#) | Maximum (#) |
| Karen Zaderej | | | | | | | | | | |
| PSU | 3/16/2022 | — | | — | | — | | | — | | 89,467 | | 134,200 | | — | | 8.20 | | 733,629 | |
| | | | | | | | | | | |
| RSU | 3/16/2022 | — | | — | | — | | | — | | — | | — | | 169,694 | | 8.20 | | 1,391,491 | |
Options (2) | 3/16/2022 | — | | — | | — | | | — | | — | | — | | 317,185 | | 10.25 | | 1,420,989 | |
| Non Equity Incentive Plan Award | — | | 673,992 | | 1,347,984 | | | — | | — | | — | | — | | — | | — | |
| Peter Mariani | | | | | | | | | | |
| PSU | 3/16/2022 | — | | — | | — | | | — | | 60,000 | | 90,000 | | — | | 8.20 | | 492,000 | |
| | | | | | | | | | | |
| RSU | 3/16/2022 | — | | — | | — | | | — | | — | | — | | 76,780 | | 8.20 | | 629,596 | |
Options (2) | 3/16/2022 | — | | — | | — | | | — | | — | | — | | 103,079 | | 10.25 | | 461,794 | |
| Non Equity Incentive Plan Award | — | | 251,492 | | 502,984 | | | — | | — | | — | | — | | — | | — | |
| Michael Donovan | | | | | | | | | | |
| PSU | 3/16/2022 | — | | — | | — | | | — | | 25,000 | | 37,500 | | — | | 8.20 | | 205,000 | |
| | | | | | | | | | | |
| RSU | 3/16/2022 | — | | — | | — | | | — | | — | | — | | 37,250 | | 8.20 | | 305,450 | |
| Options | 3/16/2022 | — | | — | | — | | | — | | — | | — | | 34,745 | | 8.20 | | 159,132 | |
| Non Equity Incentive Plan Award | — | | 174,597 | | 349,194 | | | — | | — | | — | | — | | — | | — | |
| Bradley Ottinger | | | | | | | | | | |
| PSU | 3/16/2022 | — | | — | | — | | | — | | 25,000 | | 37,500 | | — | | 8.20 | | 205,000 | |
| RSU | 3/21/2022 | — | | — | | — | | | — | | — | | — | | 44,973 | | 8.20 | | 368,779 | |
| Options | 3/21/2022 | — | | — | | — | | | — | | — | | — | | 41,948 | | 8.20 | | 192,122 | |
| Non Equity Incentive Plan Award | — | | 194,767 | | 389,534 | | | — | | — | | — | | — | | — | | — | |
| Angelo Scopelianos, Ph.D. | | | | | | | | | | |
| PSU | 3/16/2022 | — | | — | | — | | | — | | 25,000 | | 37,500 | | — | | 8.20 | | 205,000 | |
| | | | | | | | | | | |
| RSU | 3/16/2022 | — | | — | | — | | | — | | — | | — | | 42,563 | | 8.20 | | 349,017 | |
| Options | 3/16/2022 | — | | — | | — | | | — | | — | | — | | 39,700 | | 8.20 | | 181,826 | |
| Non Equity Incentive Plan Award | — | | 203,425 | | 406,850 | | | — | | — | | — | | — | | — | | — | |
| Eric Sandberg | | | | | | | | | | |
| PSU | 3/16/2022 | — | | — | | — | | | — | | 40,000 | | 60,000 | | — | | 8.20 | | 328,000 | |
| RSU | 3/16/2022 | — | | — | | — | | | — | | — | | — | | 62,910 | | 8.20 | | 515,862 | |
Options (2) | 3/16/2022 | — | | — | | — | | | — | | — | | — | | 60,576 | | 8.20 | | 496,723 | |
| Non Equity Incentive Plan Award | — | | 183,820 | | 367,640 | | | — | | — | | — | | — | | — | | — | |
(1) The table below provides the Grant Fair Value of PSU awards at the Target Value and Maximum Value: | | | | | | | | | | | | | | |
| Grant Date Fair Value of PSU |
| | Target Value ($) | | Maximum Value ($) |
Karen Zaderej | | 733,629 | | | 1,100,444 | |
Peter Mariani | | 492,000 | | | 738,000 | |
Michael Donovan | | 205,000 | | | 307,500 | |
| Bradley Ottinger | | 205,000 | | | 307,500 | |
Angelo Scopelianos, Ph.D. | | 205,000 | | | 307,500 | |
| Eric Sandberg | | 328,000 | | | 820,000 | |
(2) Stock options issued on March 16, 2022 to Ms. Zaderej, Mr. Mariani and Mr. Sandberg were premium-priced with an exercise price 25% greater than the closing market price on the date of grant.
Outstanding Equity Awards at 2022 Fiscal Year End
The following tables summarize the equity awards granted to our named executive officers that remain outstanding as of December 31, 2022.
. | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Option Awards | | Stock Awards |
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable(1) | Number of securities Underlying Unexercised Options (#) Unexercisable (1) | | Option Exercise Price ($) | Option Expiration Date | | Number of Shares or Units of Stock That Have Not Vested (#)(2) | Market Value of Shares of Units of Stock That Have Not Vested ($)(3) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#)(2) | Equity Incentive Plan Awards: Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(3) |
| Karen Zaderej | | | | | | | | | |
| 12/29/2016 | 157,125 | | — | | | 8.95 | | 12/29/2026 | | — | | — | | — | | — | |
| 12/18/2017 | 75,000 | | — | | | 27.00 | | 12/28/2027 | | — | | — | | — | | — | |
| 12/27/2018 | 59,700 | | — | | | 19.17 | | 12/27/2028 | | — | | — | | — | | — | |
| 3/16/2020 | 50,125 | | 30,075 | | | 8.61 | | 3/16/2030 | | 25,950 | | 258,981 | | — | | — | |
| 7/17/2020 | — | | — | | | — | | — | | | — | | — | | 20,033 | | 199,929 | |
| 3/16/2021 | — | | 76,600 | | | 20.91 | | 3/16/2031 | | 50,000 | | 499,000 | | 110,500 | | 1,102,790 | |
3/16/2022(4) | — | | 317,185 | | | 10.25 | | 3/16/2032 | | 169,694 | | 1,693,546 | | 89,467 | | 892,881 | |
| Peter Mariani | | | | | | | | | |
3/1/2016 (5) | 125,636 | | — | | | 5.04 | | 3/01/2023 | | — | | — | | — | | — | |
| 12/29/2016 | 110,000 | | — | | | 8.95 | | 12/29/2026 | | — | | — | | — | | — | |
| 12/18/2017 | 45,000 | | — | | | 27.00 | | 12/18/2027 | | — | | — | | — | | — | |
| 12/27/2018 | 41,800 | | — | | | 19.17 | | 12/27/2028 | | — | | — | | — | | — | |
| 03/16/2020 | 26,875 | | 16,125 | | | 8.61 | | 3/16/2030 | | 11,000 | | 109,780 | | — | | — | |
| 07/17/2020 | — | | — | | | — | | — | | | — | | — | | 3,366 | | 33,593 | |
| 03/16/2021 | — | | 61,000 | | | 20.91 | | 3/16/2031 | | 30,500 | | 304,390 | | 30,500 | | 304,390 | |
3/16/2022(4) | — | | 103,079 | | | 10.25 | | 3/16/2032 | | 76,780 | | 766,264 | | 60,000 | | 598,800 | |
| Michael Donovan | | | | | | | | | |
| 12/29/2016 | 25,000 | | — | | | 8.95 | | 12/29/2026 | | — | | — | | — | | — | |
| 12/18/2017 | 9,000 | | — | | | 27.00 | | 12/18/2027 | | — | | — | | 50,000 | | 499,000 | |
| 12/18/2018 | 18,500 | | — | | | 19.17 | | 12/27/2028 | | — | | — | | — | | — | |
| 3/16/2020 | 15,000 | | 9,000 | | | 8.61 | | 3/16/2030 | | 6,000 | | 59,880 | | — | | — | |
| 7/17/2020 | — | | — | | | — | | — | | | — | | — | | 2,333 | | 23,283 | |
| 3/16/2021 | — | | 23,500 | | | 20.91 | | 3/16/2031 | | 11,800 | | 117,764 | | 11,800 | | 117,764 | |
| 3/16/2022 | — | | 34,745 | | | 8.20 | | 3/16/2032 | | 37,250 | | 371,755 | | 25,000 | | 249,500 | |
| Bradley Ottinger | | | | | | | | | |
| 06/01/2020 | 41,875 | | 25,125 | | | 9.72 | | 6/01/2030 | | 15,500 | | 154,690 | | — | | — | |
| 07/17/2020 | — | | — | | | — | | — | | | — | | — | | — | | — | |
| 03/16/2021 | — | | 23,500 | | | 20.91 | | 3/16/2031 | | 16,927 | | 168,931 | | 11,800 | | 117,764 | |
| 03/16/2022 | — | | 41,948 | | | 8.20 | | 3/16/2032 | | 44,973 | | 448,831 | | 25,000 | | 249,500 | |
| Angelo Scopelianos, Ph.D. | | | | | | | | | |
| 09/04/2018 | 40,000 | | — | | | 45.00 | | 9/04/2028 | | — | | — | | — | | — | |
| 12/27/2018 | 32,900 | | — | | | 19.17 | | 12/27/2028 | | — | | — | | 11,681 | | 116,576 | |
| 03/16/2020 | 15,000 | | 9,000 | | | 8.61 | | 3/16/2030 | | 11,000 | | 109,780 | | — | | — | |
| 07/17/2020 | — | | — | | | — | | — | | | — | | — | | 2,333 | | 23,283 | |
| 03/16/2021 | — | | 34,000 | | | 20.91 | | 3/16/2031 | | 16,500 | | 164,670 | | 11,800 | | 110,566 | |
| 03/16/2022 | — | | 39,700 | | | 8.20 | | 3/16/2022 | | 42,563 | | 424,779 | | 25,000 | | 249,500 | |
Eric Sandberg (5) | | | | | | | | | |
(1)Stock options are fully vested four years from the option grant dated based upon a vesting schedule whereby 50% of the aggregate shares vest on the second anniversary of the option grant date with an additional 12.5% of aggregate shares vesting every six months thereafter. Prior to 2017, the options granted were based upon a vesting schedule whereby 25% of the aggregate shares vested on the first anniversary of the option grant date with an additional 12.5% of aggregate shares vested every six months thereafter.
(2)Unvested shares and units are comprised of (i) PSUs granted in 2017, 2020, 2021 and 2022, (ii) RSUs granted in 2017, 2018, February 2020, June 2020, March 2021 and March 2022 and (iii) the 2018 BLA PSU Agreements. The vesting terms for each type of award are described below:
a.PSUs - Each PSU represents the Company’s commitment to issue one share of common stock at a future date, subject to certain eligibility, performance, vesting and other conditions set forth in the respective plan and award agreement. All shares are subject to satisfaction of certain performance criteria. Upon certification of the Compensation Committee that the performance criteria is achieved, one-third of the shares are immediately vested with the shares vesting in equal annual installments each year thereafter, provided that the particular officer has been continuously employed through each vesting date as to the particular number of shares vesting. In the event of a “Change in Control” (as defined in the respective award agreement), all or a portion of the PSUs shall accelerate.
b.RSUs - Each RSU represents the Company’s commitment to issue one share of common stock at a future date, subject to certain eligibility, vesting and other conditions set forth in the respective plan and award agreement. All shares of Axogen common stock underlying the RSUs will be fully vested on the fourth anniversary of the grant date, based upon a vesting schedule whereby 50% of the aggregate shares vest on second anniversary of the grant date and an additional 25% of the aggregate shares vest each 12 months thereafter. In the event of a “Change in Control” (as defined in the award agreement) of the Company, all of the RSUs shall accelerate and become fully vested.
c.2018 BLA PSU Agreements - Each 2018 BLA PSU represents the Company’s commitment to issue one share of common stock at a future date, subject to certain eligibility, performance, vesting and other conditions set forth in the 2010 stock incentive plan and 2018 BLA PSU Agreements. Dr. Scopelianos was the only named executive officer to receive the BLA PSUs.
(3)The market value of unvested shares and units is based on the closing price of our common stock on the last day of fiscal year 2022, which was $9.98 and, with respect to PSUs, assumes target performance.
(4)Stock options issued on March 16, 2022, to Ms. Zaderej, Mr. Mariani and Mr. Sandberg were premium-priced with an exercise price 25% greater than the closing market price on the date of grant.
(5)Mr. Mariani exercised his March 1, 2016, stock options during the period of January 2023 through February 2023
(6)As of December 31, 2022, there were no equity awards outstanding for Mr. Sandberg.
Option Exercises and Stock Vested
The following provides information regarding the exercise of stock options by our named executive officers and vesting of stock awards held by our named executive officers, during 2022.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Option Awards | | Stock Awards |
| | Number of Shares Acquired on Exercise (#) | | Value Realized on Exercise ($)(1) | | Shares Acquired on Vesting (#) | | Value Realized on Vesting ($)(1) |
| Karen Zaderej | | 26,746 | | | 150,313 | | | 66,404 | | | 566,385 | |
| Peter Mariani | | 19,364 | | | 114,054 | | | 20,492 | | | 174,283 | |
| Michael Donovan | | 15,000 | | | 30,750 | | | 10,396 | | | 88,309 | |
| Bradley Ottinger | | — | | — | | | 18,063 | | | 165,012 | |
| Angelo Scopelianos | | — | | — | | | 16,046 | | | 134,827 | |
| Eric Sandberg | | 12,000 | | | 33,839 | | | 12,939 | | | 109,302 | |
(1)Based upon the closing price as reported by Nasdaq of our Common Stock at the exercise date or vesting date, as applicable.
Potential Payments Upon Termination or Change in Control
In this section, we described payments that may be made to our named executive officers upon several events of termination, including termination in connection with a Change in Control.
Employment Agreements
Axogen Corporation, a wholly-owned subsidiary of the Company, is a party to employment agreements with each of (i) Karen Zaderej, effective October 15, 2007, as amended September 29, 2011 and as further amended and restated on November 1, 2020; (ii) Peter Mariani, effective February 25, 2016, as amended and restated on November 1, 2020; (iii) Michael Donovan, effective January 13, 2011, as amended and restated on November 1, 2020; (iv) Bradley Ottinger effective June 1, 2020 as amended and restated on November 1, 2020; (v) Angelo Scopelianos, effective September 4, 2018, as amended and restated on November 20, 2020 and as further amended and restated on January 4, 2021 and (vi) Eric Sandberg effective January 22, 2019, as amended and restated on November 1, 2020.
Axogen Corporation employs Ms. Zaderej on an "at will" basis. In the event that termination occurs without "Substantial Cause" (as defined below) upon or within 365 days following a "Change in Control" (as defined below) (or 90 days preceding a Change in Control), or for "Good Reason" (as defined below) following a Change in Control, Ms. Zaderej will receive: (a) a separation payment of twenty-four (24) months of base salary paid in a lump sum within 60 days; (b) an amount equal to 200% of any bonuses or commissions paid to Ms. Zaderej during the twelve (12) months prior to her termination of employment; (c) payment of premiums for Ms. Zaderej and her covered dependents’ COBRA for the first eighteen (18) months of the COBRA continuation period; and (d) the full acceleration of the vesting of all of Ms. Zaderej’s equity awards.
In the event that termination occurs in the absence of a Change in Control, Ms. Zaderej will receive (a) a separation payment of twelve (12) months base salary paid in a lump sum within 60 days; (b) an amount equal to 100% of any bonuses or commissions paid to Ms. Zaderej during the year prior to her termination of employment; and (c) payment of premiums for Ms. Zaderej and her covered dependents’ COBRA for the first twelve (12) months of the COBRA continuation period.
Under their respective employment agreements, each of Messrs. Mariani, Donovan, Ottinger and Dr. Scopelianos is employed by Axogen Corporation on an “at will” basis.
In the event Messrs. Mariani, Donovan, Ottinger and Dr. Scopelianos are terminated without Substantial Cause upon or within 365 days following a Change in Control (or 90 days preceding a Change in Control), or for Good Reason following a Change in Control, each is entitled to: (a) a separation payment of eighteen (18) months of base salary paid in a lump sum within 60 days; (b) an amount equal to 150% of any bonuses or commissions paid during the year prior to termination of employment; and (c) payment of premiums for themselves and their covered dependents’ COBRA for the first eighteen (18) months of the COBRA continuation period.
In the event Messrs. Mariani, Donovan, Ottinger and Dr. Scopelianos are terminated in the absence of a Change in Control, each will receive, among other things, (a) a separation payment of twelve (12) months base salary paid in a lump sum within 60 days; (b) an amount equal to 100% of any bonuses or commissions paid during the year prior to termination of employment and (c) a payment of premiums for themselves and their covered dependents’ COBRA for the first twelve (12) months of the COBRA continuation period.
Messrs. Mariani, Donovan, Ottinger and Dr. Scopelianos are also entitled to have the Company pay the premiums for their COBRA (i) for the first twelve (12) months of the COBRA continuation period, or (ii) until such time as they obtain new employment that provides reasonable and comparable healthcare coverage (including without limitation, coverage of dependents), whichever period is shorter.
For purposes of each of Ms. Zaderej’s, Messrs. Mariani’s, Donovan's, Ottinger's, and Dr. Scopelianos’s employment agreements, “Change in Control” means the occurrence of any of the following events:
•any person who holds less than 20% of the combined voting power of the securities of Axogen Corporation or Axogen, becomes the beneficial owner, directly or indirectly, of securities of Axogen Corporation or Axogen, representing 50% or more of the combined voting power of the securities of Axogen Corporation or Axogen then outstanding;
•during any period of 24 consecutive months, individuals who at the beginning of such period constitute all members of the Board of Directors cease, for any reason, to constitute at least a majority of our Board of Directors, unless the election of each director who was not a director at the beginning of the period was either nominated for election by, or was approved by a vote of, at least two-thirds of the directors then still in office who were directors at the beginning of the period;
•Axogen Corporation or Axogen consolidates or merges with another company and Axogen Corporation or Axogen is not the continuing or surviving corporation; provided, however, that any consolidation or merger whereby Axogen continues as the majority holder of Axogen Corporation securities or a merger or consolidation of Axogen Corporation and Axogen will not constitute a Change in Control;
•shares of Axogen Corporation’s or Axogen’s common stock are converted into cash, securities, or other property (other than by a merger set forth above) in which the holders of the Axogen Corporation’s or Axogen’s common stock immediately prior to the merger have the same proportionate ownership of common stock of the surviving corporation as immediately after the merger;
•Axogen Corporation or Axogen sells, leases, exchanges, or otherwise transfers all or substantially all of its assets (in one transaction or in a series of related transactions); or
•the holders of Axogen’s common stock approve a plan or proposal for the liquidation or dissolution of Axogen Corporation or Axogen.
The employment agreements of Ms. Zaderej, Messrs. Mariani, Donovan, Ottinger and Dr. Scopelianos do not provide for Section 280G gross up payments.
For purposes of Ms. Zaderej’s, Messrs. Mariani’s, Donovan's, Ottinger's and Dr. Scopelianos’s employment agreements, “Substantial Cause” means:
•Commission of any act of fraud, theft, or embezzlement;
•material breach of the employment agreement, provided that Axogen Corporation shall have first delivered to the executive officer written notice of the alleged breach, specifying the exact nature of the breach in detail, and provided, further, that the executive officer shall have failed to cure or substantially mitigate such breach within ten days after receiving such written notice;
•material failure to adhere to Axogen Corporation’s corporate codes, policies or procedures which have been adopted in good faith for a valid business purpose as in effect from time to time;
•the failure to meet reasonable performance standards as determined by Axogen Corporation; or
•commission or conviction of any felony, or of any misdemeanor involving moral turpitude, or entry of a plea of guilty or nolo contendere to any felony or misdemeanor.
For purposes of Ms. Zaderej’s and Messrs. Mariani’s, Donovan's, Ottinger's and Dr. Scopelianos’ employment agreements, “Good Reason” means the occurrence of any one or more of the following:
•the assignment of any duties inconsistent in any respect with such executive officer’s position (including status, offices, titles, and reporting requirements), authorities, duties, or other responsibilities as in effect immediately prior to a Change in Control or any other action by Axogen that results in a material diminishment in such position, authority, duties, or responsibilities, other than an insubstantial and inadvertent action which is remedied by Axogen after receipt of notice thereof given by the executive officer;
•a material reduction by Axogen Corporation in the person’s base salary absent Substantial Cause; or
•the executive is required to perform a substantial portion of their duties at a facility that is more than 50 miles from the facility for which the executive performed a substantial portion of his or her duties immediately prior to the Change in Control.
2022 Potential Payments Upon Termination or Change in Control
In connection with a termination of employment, including if there is a termination in connection with a Change in Control of the Company, our named executive officers would be eligible to receive certain payments, benefits and treatment of the various forms of equity that such named executive officer holds (provided, in some cases, that certain conditions are met).
The amounts that the named executive officers would receive are set forth below based on the termination scenarios discussed above.
In accordance with SEC rules, we have used certain assumptions in determining the amounts shown. We have assumed that the termination of employment or Change in Control occurred on December 31, 2022, and that the value of a share of common stock on that day was $9.88, the closing price on Nasdaq on December 31, 2022, the last trading day of 2022.
Contractual provisions relating to cash severance are set forth above under “Employment Agreements.” With respect to the treatment of outstanding equity awards upon a termination or Change in Control, the treatment is as follows. For terminations not in connection with a Change in Control, unvested stock options, restricted stock units and performance stock units do not accelerate and are forfeited. Upon a Change in Control, unless assumed, continued or substituted (and in the case of stock options issued under our 2010 stock incentive plan, in the event that within twelve (12) months following the Change in Control, the employee is terminated without Substantial Cause or leaves the Company for Good Reason), stock options shall automatically accelerate and, become fully exercisable. Upon a Change in Control (unless it was issued under our 2019 stock incentive plan and was assumed, continued or substituted), restricted stock units become fully-vested and nonforfeitable upon the Change in Control; and performance stock units (unless it was issued under our 2019 stock incentive plan and was assumed, continued or substituted), prior to the end of the applicable performance period, become fully vested upon a Change in Control based on the greater of: (i) target performance or (ii) the expected performance as determined by the Compensation Committee. Amounts shown in the tables below for performance stock units are based on target performance. All performance stock units earned but not vested will vest immediately prior to the consummation of the Change in Control. Amounts shown under stock options, restricted stock units and performance stock units reflect the value based upon the December 31, 2022, stock price of $9.88 for the option, restricted stock unit or performance stock unit as to which vesting will be accelerated upon the occurrence of the Change in Control or termination event.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Karen Zaderej | | | | | | | | |
| | Qualified | | Qualified | | Upon | | |
| | Termination | | Termination | | Change in | | |
| | Prior to | | After | | Control | | |
| | Change in | | Change in | | Without | | Upon |
| Payment Type | | Control | | Control | | Termination | | Retirement3. |
| Severance | | $ | 1,173,339 | | | $ | 2,346,678 | | | $ | — | | | $ | — | |
| Health and Welfare Benefits | | $ | 20,371 | | | $ | 30,557 | | | $ | — | | | $ | — | |
Stock Options | | $ | — | | | $ | 41,203 | | | $ | — | | | $ | — | |
Restricted Stock Units | | $ | — | | | $ | 2,451,527 | | | $ | — | | | $ | 1,693,546 | |
Performance Stock Units | | $ | — | | | $ | 2,195,600 | | | $ | 2,195,600 | | | $ | 892,881 | |
| TOTAL | | $ | 1,193,710 | | | $ | 7,065,565 | | | $ | 2,195,600 | | | $ | 2,586,427 | |
1.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Peter Mariani | | | | | | | | |
| | Qualified | | Qualified | | Upon | | |
| | Termination | | Termination | | Change in | | |
| | Prior to | | After | | Control | | |
| | Change in | | Change in | | Without | | Upon |
| Payment Type | | Control | | Control | | Termination | | Retirement |
| Severance | | $ | 644,109 | | | $ | 966,163 | | | $ | — | | | $ | — | |
| Health and Welfare Benefits | | $ | 28,808 | | | $ | 42,012 | | | $ | — | | | $ | — | |
Stock Options | | $ | — | | | $ | 22,091 | | | $ | — | | | $ | — | |
Restricted Stock Units | | $ | — | | | $ | 1,180,434 | | | $ | — | | | $ | — | |
Performance Stock Units | | $ | — | | | $ | 936,783 | | | $ | 936,783 | | | $ | — | |
| TOTAL | | $ | 672,917 | | | $ | 3,147,483 | | | $ | 936,783 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Michael Donovan | | | | | | | | |
| | Qualified | | Qualified | | Upon | | |
| | Termination | | Termination | | Change in | | |
| | Prior to | | After | | Control | | |
| | Change in | | Change in | | Without | | Upon |
| Payment Type | | Control | | Control | | Termination | | Retirement |
| Severance | | $ | 478,308 | | | $ | 597,885 | | | $ | — | | | $ | — | |
| Health and Welfare Benefits | | $ | 20,371 | | | $ | 25,464 | | | $ | — | | | $ | — | |
Stock Options | | $ | — | | | $ | 74,176 | | | $ | — | | | $ | — | |
| Restricted Stock Units | | $ | — | | | $ | 549,399 | | | $ | — | | | $ | — | |
Performance Stock Units | | $ | — | | | $ | 889,547 | | | $ | 889,547 | | | $ | — | |
| TOTAL | | $ | 498,679 | | | $ | 2,136,471 | | | $ | 889,547 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Bradley Ottinger | | | | | | | | |
| | Qualified | | Qualified | | Upon | | |
| Termination | Termination | | Change in | | |
| Prior to | After | | Control | | |
| Change in | Change in | | Without | | Upon |
| Payment Type | Control | Control | | Termination | | Retirement |
| Severance | | $ | 533,698 | | | $ | 800,547 | | | $ | — | | | $ | — | |
| Health and Welfare Benefits | | $ | 16,664 | | | $ | 24,996 | | | $ | — | | | $ | — | |
| Stock Options | | $ | — | | | $ | 81,200 | | | $ | — | | | $ | — | |
| Restricted Stock Units | | $ | — | | | $ | 772,452 | | | $ | — | | | $ | — | |
| Performance Stock Units | | $ | — | | | $ | 367,264 | | | $ | 367,264 | | | $ | — | |
| TOTAL | | $ | 550,362 | | | $ | 2,046,459 | | | $ | 367,264 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Angelo Scopelianos | | | | | | | | |
| | Qualified | | Qualified | | Upon | | |
| | Termination | | Termination | | Change in | | |
| | Prior to | | After | | Control | | |
| | Change in | | Change in | | Without | | Upon |
| Payment Type | | Control | | Control | | Termination | | Retirement |
| Severance | | $ | 558,272 | | | $ | 837,408 | | | $ | — | | | $ | — | |
| Health and Welfare Benefits | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
Stock Options | | $ | — | | | $ | 82,996 | | | $ | — | | | $ | — | |
Restricted Stock Units | | $ | — | | | $ | 699,229 | | | $ | — | | | $ | — | |
Performance Stock Units | | $ | — | | | $ | 507,124 | | | $ | 507,124 | | | $ | — | |
| TOTAL | | $ | 558,272 | | | $ | 2,126,757 | | | $ | 507,124 | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Eric Sandberg | | | | | | | 10 | |
| | Qualified | | Qualified | | Upon | | |
| | Termination | | Termination | | Change in | | |
| | Prior to | | After | | Control | | |
| | Change in | | Change in | | Without | | Upon |
| Payment Type | | Control | | Control | | Termination | | Retirement |
Severance(1) | | $ | 549,320 | | | $ | — | | | $ | — | | | $ | — | |
| Health and Welfare Benefits | | $ | 22,645 | | | $ | — | | | $ | — | | | $ | — | |
Stock Options | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
Restricted Stock Units | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
Performance Stock Units | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| TOTAL | | $ | 571,965 | | | $ | — | | | $ | — | | | $ | — | |
1.Severance includes accrued vacation pay of $28,280
CEO Pay Ratio Disclosure
As required by the Dodd-Frank Wall Street Reform and Consumer Protection Act and Regulation S-K promulgated under the Exchange Act, we are providing the following information about the relationship of the annual total compensation of our CEO and the annual total compensation of our employees for fiscal year 2022 (our “CEO pay ratio”). Our CEO pay ratio information is a reasonable good faith estimate calculated in a manner consistent with Item 402(u) of Regulation S-K.
For fiscal year 2022, the annual total compensation for the median employee of the Company (other than our CEO) was $177,524 and the annual total compensation of our CEO was $4,398,292. Based on this information, for fiscal year 2022, the ratio of the annual total compensation of our CEO to the annual total compensation of the median employee was 25:1.
We identified our median employee from among our employees as of December 31, 2022, the last day of our fiscal year. We did not use the same median employee used in our disclosure for the fiscal year ended December 31, 2021, due to a change in the make-up of our employees as a whole. We felt it to be a more accurate representation and better metric for purposes of this disclosure to use a new median employee, identified based on estimated annual base pay, incentive compensation and grant date fair value of equity granted to each of our employees as of December 31, 2022. We then calculated the elements of the identified median employee’s compensation for 2022 in accordance with the requirements of Item 402(c)(2)(x) of Regulation S-K, resulting in annual total compensation in the amount of $177,524. With respect to the annual total compensation of our CEO, in accordance with SEC rules, we included the amount reported for Ms. Zaderej in the “Total” column for 2022 in the Summary Compensation Table. We did not make any cost-of-living adjustments in identifying the median employee. Compensation amounts were determined from our human resources and payroll systems of record.
Compensation Committee Interlocks and Insider Participation
None of our executive officers serves as a member of the board of directors or compensation committee, or other committee serving an equivalent function, of any other entity that has one or more of its executive officers serving as a member of our Board of Directors or Compensation Committee.
DIRECTOR COMPENSATION
Our Compensation Committee reviews and makes recommendations to our Board of Directors regarding compensation to be paid to our non-employee directors. For the fiscal year 2022, each non-employee director received a quarterly cash retainer payment of $10,000, with the Lead Director receiving an additional quarterly cash retainer payment of $6,875 for services to the Company, which cash payment is paid in advance each quarter. The quarterly non-Chairman committee member retainers are $2,500 for the Audit Committee, $1,875 for the Compensation Committee, $1,250 for the Governance, Nominating and Sustainability Committee and $1,250 for the Quality, Compliance and Portfolio Committee. The Chairman of the Audit Committee receives a quarterly retainer of $5,000, the Chairman of the Compensation Committee a quarterly retainer of $3,750, the Chairman of the Governance, Nominating and Sustainability Committee a quarterly retainer of $2,500 and the Chairman of the Quality, Compliance and Portfolio Committee received a quarterly retainer of $2,500. Newly elected directors receive a non-qualified stock option grant to purchase shares of the Company’s common stock with an equity value of approximately $275,000 to be issued at an exercise price equal to the fair market value of our shares of common stock on the date of grant, which option shall vest in three equal annual installments. Each calendar year on the day after election or re-election, all non-employee directors will receive an annual equity grant valued at $120,000 to be issued at an exercise price equal to the fair market value of our shares of common stock on the date of the grant, which equity shall be issued as to one half of the value as non-qualified stock option grant and the remaining half of the value as restricted stock units, which options and restricted stock units will vest one year from the anniversary of the date of the grant. Such stock options are for a term of ten years.
On December 12, 2022, our Compensation Committee approved the following increases to our directors' compensation upon guidance from our independent compensation consultant, Aon: (i) $10,000 for the annual cash retainer for each Board member; (ii) $12,500 for the lead director; and (iii) $30,000 for each director's annual equity grant.
We also reimburse our directors for travel related expenses.
The following table sets forth information concerning the compensation of our non-employee directors during the year ended December 31, 2022. Ms. Zaderej is a named executive officer who does not receive additional compensation for her service as a director, and therefore is not included in the table below.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Fees Earned | | Stock | | | | All Other | | |
| | or Paid in | | Awards | | Option | | Compensation | | |
| Name | | Cash ($) | | ($)(1) | | Awards($)(1) | | ($) | | Total ($) |
| Amy Wendell | | 85,000 | | | 60,004 | | | 60,000 | | | — | | | 206,004 | |
William Burke (2) | | 28,333 | | | — | | | 274,999 | | | — | | | 303,332 | |
| Gregory Freitag | | 45,000 | | | 60,004 | | | 60,000 | | | — | | | 165,004 | |
Guido Neels (3) | | 58,819 | | | 60,004 | | | 60,000 | | | — | | | 180,698 | |
| Alan Levine | | 55,625 | | | 60,004 | | | 60,000 | | | — | | | 175,629 | |
| Paul Thomas | | 56,042 | | | 60,004 | | | 60,000 | | | — | | | 176,046 | |
| John Johnson | | 61,806 | | | 60,004 | | | 60,000 | | | — | | | 181,810 | |
Joseph Tyndall, M.D. (4)(7) | | 2,201 | | | — | | | 274,999 | | | 14,429 | | | 291,629 | |
Mark Gold, M.D. (5) | | 51,528 | | | 60,004 | | | 60,000 | | | — | | | 172,782 | |
Quentin Blackford(6) | | 16,877 | | | — | | | — | | | — | | | 19,377 | |
1.The amounts in this column are calculated based on the aggregate grant date fair value computed in accordance with Accounting Standards Codification (“ASC”) Topic 718 as of December 31, 2022. For information regarding assumptions underlying the valuation of equity awards, see Note 11 of the Consolidated Financial Statements in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed on March 14, 2023.
2.Mr. Burke was appointed to the Board effective as of July 11, 2022.
3.Mr. Neels is a managing partner of Essex Woodlands Management ("EW") and is EW’s director nominee pursuant to the Stock Purchase Agreement, dated March 26, 2015, between the Company and EW. Cash fees earned by Mr. Neels as a director were paid to Essex through March 31, 2022, while equity awards are retained by Mr. Neels.
4.Dr. Tyndall was appointed to the Board effective as of December 13, 2022.
5.Dr. Gold retired from the Board effective as of December 13, 2022.
6.Mr. Blackford resigned from the Board effective as of March 30, 2022.
7.This represents other compensation paid to Dr. Tyndall for non-director services prior to his appointment to the board.
Non- Employee Director Stock Ownership Guidelines
The Board of Directors has adopted the non-employee director equity ownership guidelines upon the recommendation of the Compensation Committee of the Board (the "Stock Ownership Guidelines").Under the Stock Ownership Guideline, each employee director is required to hold equity interest with respect to Company's common stock with a value of equal to a least three (3) times their retainer. In February 2022, the Board of Directors amended the forms of equity interests included in the guidelines to exclude the "In-The-Money" value of vested stock option awards and include the value of unvested Restricted Stock Units. Unearned performance stock units remain excluded from the policy. Each non-employee director is required to achieve the applicable level of ownership within five (5) years of the later of the date the Stock Ownership Guidelines were amended or the date they were initially designated as a non-employee director of the Company. If a non-employee director falls out of compliance, they have one (1) year to increase their equity ownership and regain compliance.
For the purposes of determining stock ownership levels, the following forms of equity interests are included: shares owned by the non-employee director or their immediate family members residing in same household or through trusts; and the value of unvested RSUs. Each of our non-employee directors as of December 31, 2022, was in compliance with the Stock Ownership Guidelines.
A current copy of the Stock Ownership Guidelines is posted on our website at http://ir.axogeninc.com/governance-docs.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of April 1, 2023, by each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our common stock, each of our directors, each of our named executive officers and all of our directors and named executive officers as a group.
Beneficial ownership is determined in accordance with the rules of the SEC. Except as otherwise noted, each shareholder named in the table has sole voting and investment power for the shares shown as beneficially owned by them, and such shares are not subject to any pledge. Shares of common stock underlying options held by a person that are currently exercisable, or exercisable within 60 days of April 1, 2023, are considered outstanding and to be beneficially owned by the person holding such option for purposes of computing such person’s percentage ownership but are not considered outstanding for the purpose of computing the percentage ownership of any other person. Percentage of ownership is based on 42,809,994 of common stock outstanding on March 31, 2023.
| | | | | | | | | | | | | | | | | | | | |
| | | | | | |
| | | Number of Shares | | Number of Shares | | |
| | | Beneficially Owned | | Underlying Options | | |
| | | (including shares | | Currently Exercisable or | | |
| | | reflected in the | | Exercisable within 60 days | | Percent of Shares |
Name of Beneficial Owner (1) | | third column)(2) | | of April 1, 2023(2) | | Outstanding (%) |
ArrowMark Colorado Holdings LLC (3) | | 3,547,787 | | | — | | | 8.29 | % |
BlackRock, Inc. (4) | | 3,159,410 | | | — | | | 7.38 | % |
| Karen Zaderej | | 1,520,587 | | | 469,571 | | | 3.51 | % |
| Gregory Freitag | | 363,613 | | | 24,030 | | | * |
| Amy Wendell | | 118,008 | | | 82,875 | | | * |
| William Burke | | — | | | — | | | * |
| John Johnson | | 9,212 | | | 9,212 | | | * |
| Alan Levine | | 65,078 | | | 53,278 | | | * |
| Guido Neels | | 105,925 | | | 54,125 | | | * |
| Paul Thomas | | 39,672 | | | 36,636 | | | * |
| Dr. Joseph Tyndall | | — | | | — | | | * |
| Peter Mariani | | 360,976 | | | 285,319 | | | * |
| Michael Donovan | | 153,100 | | | 82,250 | | | * |
| Bradley Ottinger | | 77,252 | | | 53,625 | | | * |
| Angelo Scopelianos | | 143,701 | | | 107,900 | | | * |
All directors, named executive officers and other executive officers as a group (16 persons) (5) | | 3,297,594 | | | 1,457,321 | | | 7.45 | % |
* Less than 1%.
(1)Unless otherwise specified, the address of each beneficial owner is c/o Axogen, Inc., 13631 Progress Blvd., Suite 400 Alachua, FL 32615.
(2)Does not include shares of common stock underlying Restricted Stock Units or Performance Stock Units subject to vesting 60 days beyond April 1, 2023.
(3)This information is based solely on a review of Schedule 13G/A filed on January 10, 2023, with the SEC by ArrowMark Colorado Holdings, LLC. The Schedule 13G/A states that ArrowMark has sole voting power with respect to 3,547,787 shares and sole dispositive powers with respect to 3,547,787 shares. The principal business address of ArrowMark Colorado Holdings LLC is 100 Fillmore Street, Suite 325, Denver, Colorado 80206.
(4)This information is based solely on a review of Schedule 13G/A filed on January 31, 2023, with the SEC by BlackRock Inc. The Schedule 13G/A states that BlackRock has sole voting power with respect to 3,103,930 shares and sole dispositive powers with respect to 3,159,410 shares. The principal business address of BlackRock Inc. is 55 East 52nd Street, New York, NY 10055.
(5)Includes 340,470 shares of common stock and 198,500 shares of common stock underlying options exercisable within 60 days of April 1, 2023, held by our other executive officers.
Equity Compensation Plan Information
The following table summarizes, with respect to the Company’s equity compensation plans, the number of shares of the Company’s common stock to be issued upon exercise of outstanding options, warrants and other rights to acquire shares of common stock, the weighted-average exercise price of these outstanding options, warrants and rights and the number of shares of common stock remaining available for future issuance under the Company’s equity compensation plans as of December 31, 2022.
| | | | | | | | | | | | | | | | | | | | |
| Plan Category | | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights | | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights ($) | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in the First Column) (1) |
Equity compensation plans approved by security holders (1) | | 6,897,993 | | | 14.00 | | | 3.374,881 |
Equity compensation plans not approved by security holders (2) | | — | | | — | | | — | |
| Total | | 6,897,993 | | | 14.00 | | | 3,374,881 | |
(1) On or before April 1, 2023, an additional 2,695,518 securities were granted or reserved, net of forfeitures, as equity compensation under the Axogen, Inc. Amended and Restated 2019 Long-Term Incentive Plan. 695,146 shares remain available for issuance as of April 1, 2023.
(2) During March 2023, the Company granted 150,000 non-qualified stock options and 75,000 restricted stock units. The awards were granted as inducements material to new employees entering into employment with the Company in accordance with Nasdaq Listing Rule 5635(c)(4).
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
Review and Approval of Related Person Transactions
In accordance with our Audit Committee Charter, our Audit Committee reviews and approves (with the concurrence of a majority of the disinterested members of our Board of Directors) any related-party and affiliated-party transactions. Our Code of Business Conduct and Ethics generally addresses such situations as to conflicts of interest and is the starting basis for disclosure and review. The Code of Business Conduct and Ethics provides that a conflict situation can arise when an employee or officer takes actions or has interests that may make it difficult to perform his or her Company work objectively and effectively. Conflicts of interest may also arise when an employee or officer, or a member of his or her family, receives improper personal benefits as a result of his or her position in the Company. Loans to, or guarantees of obligations of, employees and officers and their family members by the Company may create conflicts of interest.
In addition, the Code of Business Conduct and Ethics provides that all related person transactions that meet the minimum threshold for disclosure in a proxy statement under the relevant SEC rules must be reported to and approved by the Audit Committee. Company officers and directors are required to bring promptly to the attention of our CFO or General Counsel any transaction or series of transactions that may result in a conflict of interest between that person and the Company. The Company CFO on a continuous basis, and annually, reviews with Company accounting personnel any situations that appear to have a conflict. Following any disclosure or discovery, our CFO or General Counsel will then review with the Chairman of our Audit Committee the relevant facts disclosed by the officer or director in question or the uncovered situation. After this review, the Chairman of the Audit Committee and the CFO or General Counsel determines whether the matter should be brought to the Audit Committee or the full Board of Directors for approval. In considering any such transaction, the Audit Committee or the Board of Directors, as the case may be, will consider various relevant factors, including, among others, the reasoning for the Company to engage in the transaction, whether the terms of the transaction are arm’s length and the overall fairness of the transaction to the Company. If a member of the Audit Committee or the Board of Directors is involved in the transaction, he or she will not participate in any of the discussions or decisions about the transaction. The transaction must be approved in advance whenever practicable, and if not practicable, must be ratified as promptly as practicable.
Related Person Transactions
Periodically, the Company may make contributions to the Global Nerve Foundation ("GNF"), a related party, due to certain executives of the Company being members of GNF's board of directors. In calendar year 2022, Bradley Ottinger, the Company's former General Counsel, Erick DeVinney, the Company's Vice President of Peripheral Nerve Science and Clinical Innovation, and Isabelle Billet, the Company's former Chief Strategy and Business Development Officer, served on the GNF's board of directors. The GNF was incorporated in 2021 exclusively for charitable, educational, and scientific purposes and qualifies under IRC 501(c)(3) as an exempt private foundation. Under its charter, the GNF engages in activities that focus on improving the awareness and care of patients with peripheral nerve injuries through grants, contributions and other appropriate means. The GNF is a separate legal entity and is not a subsidiary of the Company; therefore, its results are not included in the accompanying consolidated financial statements. The Company's Board of Directors authorized the Company to contribute $0.7 million to the GNF during the year ended December 31, 2022, no amounts were contributed previously.
Director Independence
Our Board of Directors currently consists of nine directors: Karen Zaderej, Gregory Freitag, John H. Johnson, Alan Levine, Guido Neels, Paul Thomas, Amy Wendell, William Burke and Dr. Adrian. Tyndall.
In determining whether our directors and director nominees are independent, we use the definition of independence provided in Rule 5605(a)(2) of the Nasdaq Stock Market’s (“Nasdaq”) Marketplace Rules. Under this definition of independence, we determined that Messrs. Burke, Johnson, Levine, Thomas and Neels, and Ms. Wendell and Dr. Tyndall are independent. Mr. Freitag and Ms. Zaderej are not independent because they serve (or in the case of Mr. Freitag, have served within the past three years) as executive officers of the Company. Each member of our Audit Committee, Compensation Committee and Governance, Nominating and Sustainability Committee also meets the heightened independence standards under the applicable Nasdaq independence rules.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
Deloitte & Touche LLP provided audit services to us and has served as our independent registered public accounting firm since March 2018. Fees for professional services provided by our independent auditors in each of the last two fiscal years, in each of the following categories are provided in the table below. Our Audit Committee has approved, pursuant to its pre-approval policies described below, all of the services listed below.
| | | | | | | | | | | | | | |
| | Deloitte & Touche, LLP |
| | 2022 | | 2021 |
Audit Fees (1) | | $ | 723,584 | | | $ | 529,434 | |
Audit-Related Fees (2) | | — | | | 15,000 | |
| Tax Fees | | — | | | — | |
| All Other Fees | | — | | | — | |
| Total Fees | | $ | 723,584 | | | $ | 544,434 | |
(1)Audit Fees consists of fees billed for professional services rendered and expenses incurred relating to the audit of our financial statements and internal control over financial reporting and the review of our interim financial statements.
(2)Audit-Related Fees consist of fees billed for the work performed in connection with our registration statements.
Our Audit Committee reviews and pre-approves the performance of all audit and non-audit accounting services to be performed by our independent registered accounting firm, other than with respect to de minimis exceptions permitted under applicable rules and regulations. All audit and audit-related services provided by Deloitte & Touche LLP during 2022 and 2021 were pre-approved by our Audit Committee.
REPORT OF THE AUDIT COMMITTEE
The Audit Committee of our Board of Directors is currently composed of the following directors: William Burke, Alan Levine and Amy Wendell, all of whom qualify as an “audit committee financial expert” under the definition promulgated by the SEC. Mr. Burke currently serves as the Chairman of the Audit Committee. The Audit Committee operates under a written charter adopted by our Board of Directors. The Audit Committee recommends to our Board of Directors, and submits for shareholder ratification, on the non-binding advisory basis, the appointment of our independent registered public accounting firm.
Management is responsible for the Company’s internal controls and the financial reporting process. Deloitte & Touche LLP is responsible for conducting an audit of our consolidated financial statements and internal controls in accordance with the standards established by the Public Company Accounting Oversight Board (“PCAOB”) and expressing an opinion on the consolidated financial statements and internal controls in accordance with GAAP. The Audit Committee’s responsibility is to monitor and oversee these processes.
The Audit Committee reports as follows:
1. Management represented to the Audit Committee that the Company’s audited consolidated financial statements were prepared in accordance with GAAP, and the Audit Committee has reviewed and discussed the Company’s audited consolidated financial statements with management and the independent auditors.
2. The Audit Committee has discussed with the independent auditors the matters required to be discussed by the applicable requirements of the PCAOB and the Commission.
3. The Audit Committee has received written disclosure and a letter from the independent accountant required by applicable requirements of the PCAOB regarding the independent accountant’s communications with the Audit Committee regarding the independent accountant’s independence and the Audit Committee concerning independence. The Audit Committee also considered whether non-audit services provided by the independent accountant during the last fiscal year were compatible with maintaining the independent accountant’s independence.
Based upon the review and discussion referred to in paragraphs 1 through 3 above, the Audit Committee had recommended to our Board of Directors that the Company’s audited consolidated financial statements be included in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on March 14, 2023.
| | | | | |
| |
| Members of the Audit Committee of the Board of Directors: |
| |
| William Burke, Chairman |
| Alan Levine |
| Amy Wendell |
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(3) Exhibits. The following documents are filed as part of this Amendment No. 1:
| | | | | | | | |
Exhibit No. | | Description of Exhibit |
31.3 | | |
31.4 | | |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | |
| AXOGEN, INC |
| /s/ Karen Zaderej |
| Karen Zaderej |
| Chief Executive Officer, President and Chairman of the Board |
| May 1, 2023 |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Karen Zaderej (with full power to act alone), as his or her true and lawful attorney-in-fact and agent, with full powers of substitution and re-substitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to the Annual Report on Form 10-K of Axogen, Inc., and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or their substitute or substitutes, lawfully do or cause to be done by virtue hereof.
In accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | |
| /s/ Karen Zaderej | | May 1, 2023 |
Karen Zaderej, Chief Executive Officer, President and Chairman of the Board
(Principal Executive Officer) | | |
| | |
| /s/ Peter J. Mariani | | May 1, 2023 |
Peter J. Mariani, Executive Vice President and Chief Financial Officer
(Principal Financial and Accounting Officer) | | |
| | |
| /s/ William P. Burke | | May 1, 2023 |
William P. Burke Director | | |
| | |
| /s/ Gregory G. Freitag | | May 1, 2023 |
Gregory G. Freitag Director | | |
| | |
| /s/ Joseph A. Tyndall | | May 1, 2023 |
Joseph A. Tyndall Director | | |
| | |
| /s/ John H. Johnson | | May 1, 2023 |
John H. Johnson Director | | |
| | |
| /s/ Alan M. Levine | | May 1, 2023 |
Alan M. Levine
Director | | |
| | |
| /s/ Guido J. Neels | | May 1, 2023 |
Guido J. Neels
Director | | |
| | |
| /s/ Paul G. Thomas | | May 1, 2023 |
Paul G. Thomas
Director | | |
| | |
| /s/ Amy Wendell | | May 1, 2023 |
Amy Wendell
Director | | |